-
A reevaluation of nystatin in prophylaxis andtreatment of oropharyngeal candidiasis
M.E. Álvarez Álvarez, A. Sánchez-Sousa and F. Baquero Department of Microbiology, Mycology Unit, Ramón y Cajal Hospital, Carretera Colmenar km 9,100, 28034 Madrid, Spain.SUMMARY
The incidence of oropharyngeal candidiasis is growing. The species of the genus Candida are extremely frequent among human colonizers. The changes in the yeast-human interaction by aging, debilitating, and immunosuppressive diseases, and the more aggressive medical interventions can explain this phenomenon. Antifungals are used both in prophylaxis and therapy, but the number of available agents remains scarce. Acquired resistance to the more commonly used antifungal agents, the azole compounds, is also an increasing threat. Policies for antifungal use should be established in order to maintain the therapeutic possibilities of the current compounds. The widespread use of systemic azoles, agents useful in deep mycosis, may increasingly exert a selective power for resistant variants. Superficial infections, such as oropharyngeal candidiasis, can be successfully controlled by nystatin, a classic polyene, which is very well tolerated and has very low rates of resistance. This review on the importance of oropharyngeal candidiasis emphasizes this therapeutic possibility, and is complemented by in vitro studies documenting the excellent activity of nystatin on both azole-susceptible and resistant strains.
Key words: Oropharyngeal candidiasis - Nystatin � ReevaluationReevaluación de la nistatina en la profilaxis y el tratamientode la candidiasis orofaríngea
RESUMEN
La candidiasis orofaríngea es una infección frecuente que ha aumentado su incidencia en la última década. Diferentes especies del género Candida son colonizadoras habituales del organismo humano. Factores como la edad, enfermedades debilitantes, inmunosupresión, utilización de antibióticos de amplio espectro durante largos periodos de tiempo y/o grandes intervenciones quirúrgicas, incrementan la incidencia de la candidiasis orofaríngea. Los antifúngicos se usan tanto en profilaxis como en terapia, aunque su número es aún escaso y la resistencia de estas levaduras, sobre todo a los azoles, ha aumentado. Por todo ello es importante establecer una correcta política en el uso de los antifúngicos, con el fin de mantener sus posibilidades terapéuticas en el futuro. El uso de tratamientos sistémicos con antifúngicos azólicos podría estar seleccionando cepas resistentes en infecciones superficiales, que pueden ser tratadas con nistatina, un polieno clásico bien tolerado y que presenta un bajo grado de resistencia. En esta revisión se pretende resaltar la importancia de esta posibilidad terapéutica, que además está avalada por estudios in vitro que demuestran la excelente actividad de la nistatina tanto en cepas resistentes como en sensibles a los azoles.
Palabras clave: Candidiasis orofaríngea - Nistatina � ReevaluaciónINTRODUCTION
Hippocrates was the first person to describe the symptoms of oral candidiasis (1), but it was Langenbeck, who in 1839, first observed a yeast (probably Candida albicans) in the oral lesions of a patient suffering from typhoid fever (2). This report is one of the first that associates the presence of a microbe with a particular lesion.
DEFINITION AND CLASSIFICATION
Oropharyngeal candidiasis is defined as an infection of both the oral cavity and the pharyngeal mucous membrane layer associated with an overgrowth of C. albicans (3, 4). This is the most frequent infection among those produced by this yeast. In 1966, Lehner (5) suggested a classification for oral candidiasis (considered as the classical classification) that has served as a basis for the following classification: acute candidiasis: acute pseudomembranous candidiasis (muguet, thrush), acute atrophic candidiasis (antibiotic related bucal pain); and, chronic candidiasis: chronic atrophic candidiasis (formerly referred to as denture stomatitis), angular cheilitis or perleche (frequently seen in association with chronic atrophic candidiasis), candidal leukoplakia or chronic hyperplastic candidiasis, median rhomboid glossitis, mucocutaneous candidiasis, and candidiasis with associated endocrinopathies.
THE PROBLEM:
THE OPPORTUNISTIC CHARACTER OF OROPHARYNGEAL CANDIDIASISThe existence of a relation between the presence of oral aphthae and severe debilitating illnesses has already been established by Hippocrates (1). In fact, C. albicans is an opportunistic pathogen that is usually present in the intestine, and probably is part of the normal flora of the cutaneous and mucocutaneous surfaces (6, 7). Using conventional culture media the presence of Candida can be documented in the oropharynx of 25% to 70% of asymptomatic and healthy individuals (8, 9), the highest rates being found among individuals with blood group O (10). In fact, saliva have fungicidal effects (11, 12), and some studies suggest that the presence of glycoproteins (blood group antigens) and salivary immunoglobulin A (IgA) may prevent adherence of Candida (13-16). In the intestinal tract microflora, and using conventional cultures, the percentage of normal individuals with positive Candida cultures varies from 50% (jejunum samples) to 60% (ileum samples) and 70% (colon) (17). This high incidence permits us to presume that it is present in the intestinal tract epithelium of most healthy individuals (18-22). The oropharynx of healthy carriers shows quantitatively low levels of colonization, between 300 to 500 CFU/ml in saliva (23, 24), depending on the time of the day (25). In some individuals, higher counts may be obtained without any correlation with oropharyngeal disease (24, 26). It is necessary to remember that the clinical diagnosis of this condition should not only be based on Candida overgrowth, but also on the clinical signs and the cytologic evidence of invasive pseudomycelia in the exfoliated epithelial cells (27, 28).
On the other hand, it is true that the particular microecological features of some individuals favor overgrowth which may facilitate subsequent oropharyngeal infection by Candida. It may be possible that even small local alterations, such as those derived from smoking tobacco or marijuana, are enough to promote Candida development in the oral cavity (29-31). However, the main factors conditioning this development seem to be changes in the normal microbial population (which could either mask/compete for receptors or interfere directly) and/or alterations of the immune system. Both of these factors can serve to explain the higher frequency of oropharyngeal colonization and infection in newborns and elderly people.
OROPHARYNGEAL COLONIZATIONAND INFECTION IN CHILDREN
The rate of oropharyngeal carriers among the pediatric population, as determined by conventional methods, is already 15% during the first week of life and goes up to nearly 50% within the following 18 months. The incidence seems to diminish from that moment, and some authors only detect a 6% presence of carriers among children older than 18 months (32). In the newbom, acute pseudomembranous candidiasis usually appears on the fourth day after birth. The incidence rate is generally between 1% and 5%, but may be as high as 18% (32, 33). This wide range of variation may reflect the human populations under study. Malnutrition and in general, low social-sanitary conditions favor oropharyngeal candidiasis (34).
OROPHARYNGEAL COLONIZATIONAND INFECTION IN ELDERLY PEOPLE
By conventional techniques, the rate of C. albicans oropharyngeal carriers among nonhospitalized elderly people is higher than 25%, and nearly 80% between those with long stays in hospital (35). A risk factor for oropharyngeal candidiasis other than the usual immunodeficiency-derived physiological status should be taken into account: the frequent drug-intake of people who reach old age. Antibiotics seem to favor Candida selection, but psychotropic drugs might also cause alterations in the oral microbial population. Moreover, the use of dentures can also lead to changes in the normal microflora (36) as they allow C. albicans to overgrow, thus giving rise to "denture stomatitis" (37-40). This may occur in over 60% of denture wearers, with a strong female predominance (41). Elderly people produce little saliva, which is responsible for both washing and pH regulatory effects. In a study among 52 elderly patients, 80.8% of whom suffered oropharyngeal candidiasis, treatment with a saliva substitute containing mucin led to an increase of the oral pH and reduced the number of candidiasis to 5.8% (42).
IMMUNOSUPPRESSED PATIENTS
In the past few years, hospitals have witnessed an increase in the number of immunodefficient patients or patients requiring potentially immunosuppressive treatments that reduce host primary response (43, 44). The topical or inhaled use of steroids is a risk factor for candidiasis (45, 46). Particularly if phagocytosis is impaired, the risk of C. albicans infection is clearly increased (47-49). Radiation therapy can result in a change in oral bacterial flora and promote colonizacion or infection (50-53).
PRIMARY IMMUNODEFICIENCIES
Primary immunodeficiencies such as neutrophil dysfunctions, cellular immunodeficiencies, humoral immunodeficiencies, complement alterations or complex combined syndromes, particularly in children between 3 and 14 years of age, can cause alterations in the oral microflora that can lead to an overgrowth of Candida and subsequent oropharyngeal inflammation (54).
PATIENTS WITH SOLID NEOPLASTIC DISEASES
In patients suffering from different types of neoplasia the rate of colonization of the oropharynx by C. albicans is extremely high, reaching almost 70% (55). Figures are very high among patients submitted to therapeutic irradiation of the oral cavity (52, 56). The frequency of Candida isolation from the oral cavity and feces of neoplastic patients who are taking wide-spectrum antibiotics may surpass 80% (57). Oropharyngeal Candida infections in this group were found in 42% of the cases (58). In pediatric oncology patients, the rate of oral complications by Candida were significantly higher among patients with a solid tumor than with patients with leukemia (13.5% vs. 4%) (58). In general, antibiotic therapy �including perioperative use of antibiotics� has a bigger influence on the oral counts of C. albicans than radiation or anticancer chemotherapy (59).
PATIENTS WITH HEMATOLOGIC DISEASES
Cancerous hematologic and neutropenic patients show high degrees of immunosuppression before or after therapy, which bears a higher risk of Candida infections (60), C. albicans being the primary causative species (61-63). Studies on neutropenic children show Candida colonization in 45% of the oral samples and at least 25% of the intestinal samples, C. albicans, C. tropicalis, C. pseudotropicalis (C. kefyr) and C. krusei being the species most commonly found (64). This may be the consequence of the important changes in the oral microflora that result from cytotoxic chemotherapy (65-67). Of patients with untreated leukemia, 20% develop oral complications (68).
TRANSPLANT PATIENTS
Organ transplant patients, particularly those with bone marrow and liver transplants, who require immunosuppressive treatments have oropharyngeal candidiasis as a very frequent complication (69, 70), which leads to prophylactic attitudes (69). In fact, the incidence of Candida colonization in liver transplant patients has reached 67% in one series (71), but was slightly lower among renal patients (72).
HIV-POSITIVE AND AIDS PATIENTS
Oral infective symptomatology is quite frequent in HIV- positive and AIDS patients (73, 74), probably due to the local environmental changes in the oral cavity (75). The most prevalent yeast pathogen is C. albicans, both in adults and in children (76). It has been suggested that HIV infection increases the adhesivity of C. albicans to the oral mucosal surface (77). Similarly, the salivary deficit in these patients may be involved in high rates of C. albicans colonization (78). The frequency of esophageal and oropharyngeal candidiasis among these patients varies between 45% and 95% (79) and is considered one of the earliest symptoms of AIDS among HIV-positive patients (80-82), as well as an early predictive marker of pulmonary tuberculosis in these patients (83). In children from HIV-positive mothers, the rate of Candida colonization and infection may be particularly high (84). The prevalence rates of oropharyngeal colonization by C. albicans on HIV-positive patients without evidence of infection range in some series from 50% (85) to 78% (20, 86). This may vary in different studies, but the lowest rate found in nonsymptomatic HIV patients was 24% (87). C. albicans has the highest frequency of isolation (55%) and its most commonly found biotype is 1 (85); the prevalence of other species appears to be lower: C. tropicalis (17%) and C. krusei (12%) (55).
DIABETIC PATIENTS
Oral candidiasis is frequent in patients with diabetes (88, 89). In these patients, the rate of Candida colonization is very high (75%), at least double the that for healthy controls (90). The colonization frequency is higher in type I than type II diabetes (91). In noninsulin-dependent diabetes, the nonsecretors have a higher risk of infection (13). The epithelial mucosal surface of the oral cavity of diabetic patients is more adhesive for C. albicans than in nondiabetic patients (92).
CLINICAL CONSEQUENCES OF OROPHARYNGEAL INFECTION
Oropharyngeal infection by Candida can give rise to different symptoms which are be related to the affected organ. Although it can occur asymptomatically (93), in most cases the patient suffers from odynophagia. If the pharynx is affected, it can cause hoarseness; if the esophagus is affected, dysphagia. When an oropharyngeal candidiasis patient refers to this symptom it can be assumed that he is suffering from esophagitis caused by Candida without an endoscopy confirmation (94, 95). When the symptomatology is extreme, the patient tends to decrease his intake of food and, if the situation is maintained, a problem of malnutrition can result. Affection of the intestinal tract as a result of a Candida infection remains in most cases a local problem; some authors consider esophageal candidiasis as a locally invasive type of illness. Esophageal affection can be followed by complications such as mucous adherences, paraesophageal abscesses, perforation and stenosis (96). Those patients who are more severely immunodepressed show a tendency towards more extended forms of the oropharyngeal infection (97).
OROPHARYNGEAL CANDIDIASISAS THE SOURCE OF INVASIVE INFECTION
Candida is an ubiquitous opportunistic pathogen due in great extent to the broad range of disease conditions it can cause, ranging from mucosal to systemic (98). Egger et al. suggest that systemic infections by Candida occur independently from oral or mucocutaneous candidiasis (99), even if both types of candidiasis can take place simultaneously in immunosuppressed patients because of their predisposing factors. It has been estimated that patients with hematologic malignancies colonized by high numbers of Candida may develop invasive candidiasis in 20% of the cases (100).
ROLE OF ANTIFUNGAL AGENTS IN ORIPHARYNGEAL CANDIDIASIS
Azole compounds
The azole-derived substances that were discovered in the 1960s are all synthetic compounds. Four azoles have been approved for the treatment of systemic fungal diseases: miconazole, ketoconazole, fluconazole and itraconazole. The imidazolic and triazolic molecules have two or three nitrogen atoms respectively. Although there are variations among compounds, these agents can be considered as broad spectrum fungistatic agents.
Mechanism of action
The azoles inhibit the synthesis of ergosterol, an essential membrane lipid in fungi, thus altering the fungal membrane. Fluconazole, for instance, inhibits a monooxygenase associated to a P450 fungal cytochrome (lanosterol, 14a-demethylase). The inhibition of this enzyme blocks the conversion from lanosterol to ergosterol, which is an essential compound in fungal lipid membrane. As a consequence, the membrane permeability is altered, the membrane-bound enzymes are deactivated, and cell replication is also prevented (101-103).
Mechanism of resistance
There are several mechanisms involved in resistance to azole compounds. The first involves the reduction of the permeability resulting from changes in the membrane sterol composition (104). The second involves modifications of the target (105, 106), in which the alteration of the target enzyme, lanosterol alfa demethylase 14a-d, was shown to be the mechanism of resistance in azole-resistant mutants (107). Azole-resistant isolates displaying an alteration(s) in target site (in general near the active site of the enzyme) remain rare, and can be divided into the two following groups: i) resistance results from inactivation of the P450 14- dm (108-110); and ii) mutations which alter the inhibitor binding but not the binding of the endogenous substrate (107). Overexpression of cytochrome P450 14-dm was the major mechanism of resistance in a pair of C. glabrata strains that developed resistance during therapy (111, 112). The third mechanism involved in azole resistance is the mutations in D5,6 sterol desaturase, another enzyme in ergosterol biosynthesis (113, 114). Altered D5,6 sterol desaturase may be an alternative explanation for resistance in a few isolates of C. albicans. D5,6 Sterol desaturase mutants block the formation of 14-methyl-3,6diol and cause the accumulation of the precursor 14-methylfecosterol which can support growth. However, C. albicans is diploid and isolates leaky defects (partially active enzyme) may exhibit variable degrees of resistance (115, 116). D5,6 Sterol desaturase mutants are also deficient in ergosterol and thus amphotericin B-resistant (2 isolates C. albicans from AIDS); this is one of the cases of cross-resistance with amphotericin B (117, 118). The fourth mechanism involves efflux (119). In C. albicans, reduced azole content in treated cells directly correlates with resistance (106, 120, 121). A correlation was found between resistance and increased mRNA levels of some pumps. This is an energy-requiring phenomenon and is now known to be mediated by one or more multidrug resistance genes, namely CDR1, CDR2 and CaMDR1. CDR1 is a transporter protein in the ABC transporter family of genes and work is now progressing on the role of CDR2, PDQ5 and other members of this transporter family in azole efflux. All licenced azoles are substrates for CDR1. CaMDR1 (Ben R) is a member of the major facilitator family and to date has been found to export only fluconazole from the cell. CDR2 plays an important role in mediating the resistance of C. albicans to azole anfifungal agents (122). The overexpression of transporter genes in C. albicans appears to be an inducible phenomenon (123), and the local environment may therefore influence the susceptibility to azole compounds. In C. krusei fluconazole-resistance may be attributable to efflux mediated by an homolog of CaMDR1 (124), which is apparently specific for fluconazole (125, 126). Efflux appears not to be the primary mechanism of itraconazole resistance, but impermeability may be important. In summary, transport-associated resistances affect different azoles in various ways. For example, fluconazole resistance may not necessarily affect itraconazole (110) or ketoconazole (127).
AZOLES IN PROPHYLAXIS OF OROPHARYNGEAL CANDIDIASIS
Prophylaxis with fluconazole at a daily dosis of 50 to 100 mg has significantly reduced the frequence of oropharyngeal candidiasis and recurrence rates in advanced status HIV-patients (128). It is also advisable for preventing muguet in AIDS and AIDS-associated complex patients (81). Both the tolerance and the efficiency of fluconazole and itraconazole as prophylactic agents for oropharyngeal candidiasis in immunocompromised patients are high (129, 130). The use of fluconazole as prophylaxis in HIV patients for long periods of time has given rise to the emergence of resistances among the yeasts which infect and colonize these patients (see below) (131).
AZOLES IN TREATMENT OF OROPHARYNGEAL CANDIDIASIS
Evaluation of more than 100 patients treated with 50 to 300 mg/day of fluconazole for 28 days revealed that between 61% and 88% of them were cured (132, 133), and that the symptoms disappeared within the first week of treatment (101, 134). Comparative studies of cancer patients with oropharyngeal candidiasis undergoing therapy with fluconazole or ketoconazole showed a similar number of therapeutic failures (4/32 treated with 100 mg/day of fluconazole, 4/36 treated with 400 mg/day of ketoconazole) (135). Studies of fluconazole (200 mg/day) for the prevention of invasive fungal infections in patients with AIDS demonstrated that during 3 years of continued use, 10.6% of patients suffered at least one episode of oral candidiasis (136). Ketoconazole and itraconazole may have a more restricted effect in advanced HIV patients, considering that these agents require gastric acid for absorption. Hitchcock et al. pinpoint the following host factors that may have an influence in the failure of the antifungal therapy: i) the degree of immunosuppression; ii) location and severity of the infection; iii) physiological alterations such as gastrointestinal malfunction or diminished saliva production; iv) pharmacokinetic parameters, such as poor oral absorption or drug interactions; and v) patient compliance (137).
Side effects
Azole antifungal agents are usually nontoxic; nevertheless, some rare cases of hepatotoxicity, particularly among patients treated with ketoconazole, have been reported (138). In a study performed with 100 mg of fluconazole a rise of the Glu-OAA-transaminases (GOT) levels was observed (139). Gearhart reported an increase of both the transaminases and the total bilirubin levels and alterations in time of prothrombin, all of which disappeared once the treatment stopped (140). Topic imidazoles are toxic at high concentrations, when they directly interact with and damage the host cell membrane (141-143).
POLYENIC ANTIBIOTICS
We are only interested in two macrolides, nystatin and amphotericin B, which have been used for more than 20 years now, out of the 60 polyenic antibiotics that have been discovered so far.
Nystatin was discovered by Hazen and Brown in 1950 (144). Extracted from mycelium of Streptomyces noursei by MeOH (7), the drug was further developed by Squibb Research Laboratones (145) and launched with the trade name of Mycostatin®. It is a tetraene polyene with 36 carbon atoms and contains a mycosamine group. Although rather hydrophobic, this powder is partially soluble in propyleneglycol and totally soluble in organic solvents (e.g., dimethyl- formamide and dimethyl-sulfoxide). It acts against many moulds and yeasts such as Candida, Torulopsis and Geotrichum with MICs ranging from 1 to 12.5 �g/ml. It was the first polyene to be used in topical treatments of cutaneous and mucocutaneous infections by Candida. The structural formula of nystatin can be seen in Figure 1.
Amphotericin B is an important drug produced by Streptomyces nodosus (146) and widely used to treat serous systemic fungal infections. According to Ducher, the producer strain was first isolated at Squibb Laboratories in 1953, and the initial reports of antifungal activity were published in 1956 (147). Thirty years later, this antifungal agent is still active at low concentrations (1 �g/ml) against most yeast and filamentous fungal pathogens. The prestige of polyene compounds remains intact. Amphotericin B is a member of the polyene macrolide class of antibiotics; it is amphoteric, forming soluble salts in both acidic and basic environments. The agluconic moiety of this heptaene-type polyenic compound, is responsible for lipophilic behavior and the molecular instability of the molecule (including photooxidation). The molecular weight is 960 and its protein binding is as high as 90%. It is not soluble in water and is solubilized by the addition of sodium desoxycholate, which, when combined with amphotericin B forms a colloidal dispersion (148, 149).
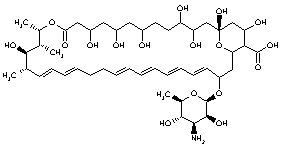
Figure 1. NystatinMechanism of action of the polyenes
The mechanism of action of nystatin, amphotericin and other polyenes is based on the formation of insoluble complexes with the sterols of the cell membrane (ergosterol in fungi and cholesterol in mammals), which causes changes in the permeability that cause K and P efflux and alteration of the proton flux leading to cell death (106). The effects of polyenic activity on the fungal cell are complex and depend on a variety of factors including cellular growth phase, doses and mode of administration (150).
It has been suggested that these effects can be divided into dose-dependent stages: stimulation, permeability and lethality. The dose requirements for stimulation are lower than those necessary to alter the permeability, whereas lethality requires much higher doses than any of the other two. Apparently, lethality does not only depend on changes in the permeability, but also on another factor. It has been found that oxidation-dependent events for which damage to the cell is associated with production of toxic oxygen derivatives might be connected to lethality of amphotericin B. Providing this, the ability of the cell to degradate such toxic products should affect its resistance to the damage caused by amphotericin B (151). Two possible mechanisms could be used by this drug to render these products. The final is amphotericin B autooxidation producing free radicals (152); in this case, the formation of these compounds would not be linked to the membrane permeability and would not depend on the kind of sterol. The second hypothesis is the amphotericin B-mediated increase of the membrane permeability (particularly to monovalent cations) which would depend on the structure of the sterol present in the membrane and suggest the possibility that there is a connection between these two mechanisms (153).
MECHANISM OF ACTION:
DIFFERENTIAL ASPECTS BETWEEN AMPHOTERICIN B AND NYSTATINAs we have seen, nystatin is similar in structure to amphotericin B, meaning therefore that their interaction with sterols should be almost identical. Nevertheless, there are small differences that allow nystatin to unspecifically bind sterols, which results in both a lower affinity for cholesterol and a relatively higher affinity for ergosterol than amphotericin B. In fact, nystatin activates potassium release (as a result of the membrane damage) more efficiently in yeast cells than in erythrocytes, which is opposite to amphotericin B. It can be concluded that the nystatin target-specificity is higher and, therefore, better than that of amphotericin B and thus, its therapeutical possibilities should be reevaluated (153). On the other hand, studies performed using kinetic fluorescence methods show that amphotericin B and nystatin might have very different activities on sterol- free egg-phosphatidylcholine unilamellar vesicles (154). In such a case, it is amphotericin B that triggers the increase of potassium permeability. This observation stresses the importance of the selective interaction of nystatin with ergosterol. In any case, nystatin antifungal intrinsic activity is slightly inferior to that of amphotericin B, despite basically sharing the same mechanism of action (153, 155).
MECHANISMS OF RESISTANCE
Despite the fact that they have been used on patients for more than 30 years, fungal resistance to nystatin or amphotericin B is exceedingly rare (156, 157). Strains of C. albicans have ocasionally been reported to be resistant to amphotericin B (MIC >=2 mg/l) (158, 159). Most resistant strains are related with immunocompromised patients on long-term therapy (160-163) associated with fungemias, with poor clinical response. Apparently, decreased amphotericin B susceptibility in Candida isolates tends to occur in AIDS patients when compared with patients with less advanced HIV disease (164). Resistance may occur in these patients with higher frequency among strains belonging to other Candida species. The in vitro/in vivo correlation of resistance with clinical success is clear in some series: in strains with amphotericin B MICs exceeding 0.8 mg/l a poor clinical outcome is the rule (165).
Several mechanisms of resistance to amphotericin B could be considered. We have already seen that the target for this drug is the fungal cell membrane ergosterol and to reach that target, the antifungal agent must go through a rigid cell wall. Little is known about the contribution of cell wall permeability to amphotericin B resistance (166). Most of the fungi resistant to these antibiotics have quantitatively or qualitatively altered cell membrane lipid composition (104), although it seems as if this single feature is not enough to make fungi amphotericin B resistant (162, 167). The emergence of resistances is due to following factors: i) a reduction in the ergosterol content of the membrane (168, 169) (eventually as a result of being substituted by more resistant sterols); and, ii) an increase of the catalase levels in C. albicans-resistant strains (which confers resistance to the oxidation-dependent damage) (13, 170), other mechanisms involving subtle modifications of the membrane structure rather than sterol substitutions (171).
Nystatin resistance
The polyenes usually present cross-resistance (172); for example, in vitro nystatin-resistant mutants with a modified sterol profile (e.g., D8-D7 isomerase mutants) are also resistant to amphotericin B. Experiments with amphotericin B low-level resistant mutants (non-sterol related membrane mutants) show that these may remain susceptible to nystatin (171), however, in clinical practice, the opposite usually occurs. In a small group of Candida isolates, in particular C. rugosa, a certain level of resistance to nystatin and an apparently higher susceptibility to amphotericin B was observed (173).
Reports on nystatin susceptibility differ according to the different techniques used. In general, the susceptibility appears to be maintained at similar rates over the years. For instance, in 1951, the MICs of nystatin against various yeasts was recorded to be in the range of 1.56 to 6.25 mg/l (174); in 1954 Drouhet verified the absence of resistant Candida strain isolates in the range of 1.56 to 12.5 mg/l (175-177); and we obtained essentially the same results nearly half a century later. Again the same result was obtained by Lombardi et al. with nystatin MIC for Candida, Cryptococcus, Saccharomyces, Geotrichum, Rhodotorula, Torulopsis and Trichosporum being 99% less than or equal to 8 mg/l (178). In some areas of the world, such as Turkey, the situation may differ. In 1993, Sürücüoglu et al. studied 100 Candida strains isolated from patients with hematologic malignancy and showed that 4% of the strains were resistant to nystatin (64). High rates of resistance were confirmed the same year by other Turkish authors with more than 20% of Candida strains (179). In Poland, in a single study, 50% of the yeast strains of clinical origin were resistant at a concentration of 10 mg/l) (180). Due to their extreme rarity, these observations should be analyzed with caution, and, if possible, confirmed by standard techniques.
CLINICAL USE IN OROPHARYNGEAL CANDIDIASIS
ProphilaxisAntifungal prophylaxis has a highly beneficial effect on the occurrence of oropharyngeal candidiasis (181). The value of nystatin as a prophylactic agent should be reviewed. It is known that the use of nystatin prophylactic reduces oropharyngeal candidiasis incidence in elderly people, immunodepressed patients, neutropenic patients and patients suffering from malignant diseases (182-184). In leukemia patients (185), bone marrow transplant recipients and neutropenic patients, nystatin associated to chlorhexidine or ofloxacin, or combined with ketoconazole and iodated povidone, reduced mucositis, gingivitis and oral infections (53, 70, 186). The effectiveness of the suspensions of nystatin and amphotericin B appears to be similar; both being equally efficacious in the oral cavity (187). Nystatin also appears to be similar in oropharyngeal candidiasis prophylaxis of neutropenic patients to ketoconazole treatment with ketoconazole (200 mg/b.i.d.) which offered almost identical results to nystatin (500,000 U/q.i.d.), i.e., incidence reduction of 14% with ketoconazole vs. 17% with nystatin; ketoconazole appears to be more effective in the prevention of invasive candidiasis (188). Nystatin was found to be as effective as clotrimazole in a randomized study on prophylaxis of oral candidiasis in orthotopic liver transplant patients (189). Nystatin appeared to be effective in delaying the onset of oropharyngeal candidiasis in 128 HIV- infected patients. It is suggested that patients with CD4+ cell counts <200 who are carriers of C. albicans and have a history of oral candidiasis may be likely to benefit from antifungal prophylaxis (190).
Treatment
In general, in uncomplicated patients, the topical use of nystatin or amphotericin B is sufficient to control oropharyngeal candidiasis (96, 191). For such a purpose, nystatin has been administered in oral suspensions, vaginal suppositories or tablets (192). In most cases the tolerance is excellent, even in compromised patients (193). Randomized assays have also proven the efficacy of denture rinses containing nystatin in the control of chronical atrophic candidiasis in elderly people (36). The nystatin solution applied to the acrylic surface of the denture prevents adherence (194) and produces a greater inhibition of C. albicans growth (195). In dentistry, nystatin is considered a very useful agent to control Candida infections (196). In children with stomatitis, the association of nystatin and lidocain is very effective in reducing both the pain and the lesions (197). A reduction in the rate of mucositis associated with head and neck radiotherapy has been obtained with nystatin combined with hydrocortisone, tetracycline and diphenylhydramine (198).
How can nystatin efficacy becompared with other antifungal agentsin the therapy of oropharyngeal candidiasis?
In a randomized study on cancer patients, nystatin and ketoconazole were similar in cure rate (21/24 vs. 13/18 patients) and erradication of the initial fungal pathogen (66% vs. 61%) (199). In oropharyngeal candidiasis of newborns, therapy with nystatin (100,000 U/ml) produced a 73% clinical cure and mycological erradication; the success rate was higher, 94%, for ketoconazole (200). Nystatin (200,000 U oral suspension q.i.d.) was also inferior to oral ketoconazole (200 mg/day) in an African study of oropharyngeal candidiasis infections in AIDS patients (201).
When fluconazolc (2-3 mg/kg/day) was compared with nystatin (400,000 q.i.d. for 14 days) in immunocompromised children, the clinical cure rate was 91% for fluconazole and 76% for nystatin; the erradication of the yeast pathogen was attained in 51% and 11% respectively (202). In general, these studies indicated that nystatin is a safe and effective agent for the treatment of oropharyngeal candidiasis, but in the infections caused by azole-susceptible yeast pathogens and at the current dose schedules, nystatin tends to be less effective than the oral azolic compounds. The pattern of interactions between the cells and the drug with topical vs. tissue-distributed compound could explain these differences.
The increase in azole-resistant yeast pathogens has reactivated the interest for a reevaluation of polyenic compounds, including both nystatin and amphotericin B, as first- line therapy for oropharyngeal candidiasis in AIDS patients (203).
Side effects
Nystatin is not administered parenterally due to its toxicity. Its scarce absorption in the digestive tract is responsible for its good oral tolerance, with very infrequent side effects; on exception it can cause digestive episodes with nausea, diarrhea and vomiting, or allergy (dermatitis) (204-206). Toxicity in cutaneous or mucosal topical use has not yet been described.
As in the case of nystatin, amphotericin B presents very little oral absorption and, subsequently, no toxicity when administered topically. Intravenously, however, it must be regarded as the most toxic antifungal drug: it causes flebitis at the injection site and renal failure at very high doses (10- 20 g) (207), as well as other negative effects such as fever, nausea, vomiting, anemia (208, 209), hypokalemia, thrombocytopenia, liver dysfunction and allergies. These effects can be reduced with the new formulations, e.g., liposomes (210-213), lipidic complexes (214) and colloidal dispersion (215), which all act in a similar way (216).
A RAPIDLY GROWING PROBLEM:
AZOLE-RESISTANT C. ALBICANS ISOLATESDevelopment of resistance to azoles
In 1978, Holt and Azmi (217) described a case of neonatal candidiasis in which resistance to miconazole developed in a strain of C. albicans. Nowadays, fluconazole resistance is more and more frequent, particularly in advanced AIDS patients (218, 219). The emergence of resistance appears to be related to low CD4+ lymphocyte counts (<50/ mm3), long-term treatments with fluconazole 50 to 100 mg/ day, maintenance therapies and prophylaxis (220-225). Insufficient doses with reduced levels of fluconazole in saliva have been considered a risk factor for selection of resistance (226). Resistance rarely appears in short-term or intermittent treatments (127); however, these resistant strains can be transmitted from AIDS patients who present C. albicans in their oral cavities (227). In fact, some studies were unable to reveal any difference in the in vitro susceptibility to imidazolic compounds (and the same was true for polyenics) when treated and untreated patients were compared (228). Some observations of this type could be attributed to the clonal dispersion of a resistant C. albicans strain. In fact, molecular epidemiology studies have shown that among the isolates obtained in recurrent candidiasis, including fluconazole-resistant strains, the same C. albicans clone is sequentially recovered (229).
Microbiological resistance vs. clinical resistance
In general, the development of clinical resistance to fluconazole is clearly correlated to the in vitro studies of susceptibility (230), and the microbiological results can be used to improve the treatments of those HIV patients suffering from oropharyngeal candidiasis and/or esophageal candidiasis (231). Using conventional culture media (RPMI, RPMI-G), therapeutic failures in this disease have been related with strains showing minimal inhibitory fluconazole concentrations �8 to 16 mg/l, and therapeutic success if MICs were <1 to 2 mg/l (231, 232-234).
Selection of non-albicans Candida with resistance to azoles
The frequent use of fluconazole in oropharyngeal candidiasis treatment of HIV-infected patients may contribute to select non-albicans species, with less intrinsic susceptibility to the drug (230, 234, 235). The emergence of these non-albicans species usually occurs after a second prophylaxis (236), particularly in bone marrow transplant and neutropenic patients (237). Interestingly, comparative studies indicate that selection of non-albicans species, such as C. glabrata, takes place in the group of patients treated with fluconazole rather than in that of patients treated with polyenes (238).
A RATIONAL POLICYFOR ANTIFUNGAL PRESCRIPTION
Should we reserve azole drugs for the treatment of deep fungal infections?
Many oral and systemic therapies have been employed in oropharyngeal candidiasis treatment, the most common being topical nystatin, clotrimazole, ketoconazole, fluconazole and itraconazole. Even though theoretically systemic antifungal azoles present certain advantages, resistance-derived problems can limit their use (156, 239). The prevention of further spread of resistance may be an advisable strategy in order to maintain the current relevant therapeutic options of azole compounds in severe fungal candidiasis. For this reason, it could be appropriate to reduce azole consumption as much as possible in order to decrease the selection pressure. This policy should be based on a substitutive use of azoles for topical polyenic compounds. For instance, it has been proposed that the treatment of neonatal oral candidiasis should be performed with nonabsorbable (e.g., polyenic) drugs, while the systemically active agents should be used primarily if a risk of dissemination exists or if widespread disease is present (240). The same approach could be applied to other groups of patients, including HIV-positive patients.
How to control and prevent the spread of azole-resistant strains
Not only restricting azole use in all those clinical pictures in which topical therapy with polyenes is possible, but also automedication and, in particular, low-dose, long-term therapy might help (241). In any case, an international surveillance program devoted to the early detection of azole- resistant geographical "hot points" could also be advisable. Such surveillance should be of a microbiological nature, and not based only on the percentage of clinical failures, which are more influenced by the type of patient and the pharmacological features of the azoles, including drug interactions (242). Where high rates of azole resistance are documented, the increase in polyenic use should serve to prevent further spread of resistances by decrease of the selective pressure. It can be expected that polyenic compounds, being equally active on fluconazole-susceptible and -resistant strains, may contribute to the elimination of the azole-resistant isolates. If it occurs at a time when the prevalence of azole-susceptible strains remains relatively high, a "healthy recolonization" by nonresistant strains may occur. It could be interesting to see if a policy of increased use of polyenic compounds could produce such a restorative process.
REEVALUATION OF POLYENESIN OROPHARYNGEAL CANDIDIASIS
Microbiological aspects
To determine the MICs, microdilution techniques were used for which the results are comparable to those obtained by NCCLS (243) macrodilution tests (244, 245), and may be even better for the prediction of the in vivo response (246). A total of 134 strains were studied: 78 C. albicans, 47 C. glabrata, 6 C. tropicalis, 1 C. pseudotropicalis, 1 C. parapsilosis, 1 C. lusitaniae, and 2 strains of the ATCC 64450 and ATCC 64458 collections. Seventy-eight oropharyngeal samples, 23 bronchial samples, 2 blood, and 31 other samples were taken.
Determination of MIC (micromethod)
Plates. Sterile plastic microplates containing 96 flat bottom wells (Costar®) were used. The fluconazole concentration ranged from 64 to 0.125 mg/l. The ketoconazole concentration ranged from 4 to 0.003 mg/l. The itraconazole and nystatin concentration ranged from 8.0 to 0.06 mg/l and amphotericin B concentration ranged from 16 to 0.03 mg/l. In each well, a 100 �l final volume was dispensed. The first column was the negative control (without yeast inocula) and the 12th column was the positive control (without antifungal agent).
Inoculum. The yeast isolates were grown in Sabouraud-chloramphenicol agar for 18 to 24 h at 35 �C. Initial suspensions were prepared from at least five colonies of each culture, in sterile 0.9% saline, and adjusted to a turbidity of 0.5 MacFarland (1-5 x 106 CFU/ml). These initial inocula were diluted 1/10 and 10 �l were added to each well (columns 2 to 12).
Medium. RPMI 1640 (Gibco® BRL) with l-glutamine, buffered with morpholinepropanesulfonic acid (MOPS) buffer (Sigma®) to a final pH 7.0 by using 10 M NaOH, this medium was supplemented with glucose 2% and casitone agar.
Antifungal agents. Fluconazole (Pfizer® S.A., Madrid, Spain), itraconazole and ketoconazole (Janssen® Farmacéutica, S.A., Madrid, Spain). Amphotericin B and nystatin (Squibb S.A.®).
Incubation. Microplates were incubated 24 h at 35 �C.
Reading. A spectrophotometric reading was made at 405 nm (MultisKan®) after 5 min agitation.
End point criteria. The MIC end point was calculated at the lowest drug concentration giving rise to an inhibition of growth equal or greater than 50% and 90% of the positive control (without antifungal agent).
Nystatin activity on Candida spp
Using the standard RPMI method (80%), a strictly monomodal distribution of MICs was observed in our collection of strains. The mode MIC was 2 mg/l, and the range 1 to 8 mg/l.
Essentially the same distribution was obtained on casitone medium, in which the activity of nystatin is higher, the mode MIC was 0.25 mg/l, and the range between 0.03 and 2 mg/l. These results strongly suggest the absence of any acquired mechanism of resistance to nystatin among our series of 121 isolates (Fig. 2).
Figure 2. Nystatin activity on Candida spp.
Figure 3. Nystatin activity against fluconazole-resistant C. albicans.Nystatin activity against fluconazole-resistant C. albicans
The nystatin MIC distribution for fluconazole-resistant strains (MIC >=8 mg/l; in most cases, >64 mg/l) remains identical to that shown for fluconazole-susceptible isolates, which reveals the absolute absence of cross-resistance between both groups on antifungals (Fig. 3).
Nystatin activity on non-albicans Candida
Again a monomodal distribution of nystatin MICs was found in the collection of 44 non-albicans Candida strains. The mode value, 2 mg/l, and the range (1 to 4 mg/l), was practically the same as for C. albicans. This result emphasizes the absence of resistance to nystatin in these strains (Fig. 4).
Figure 4. Nystatin activity on non-albicans Candida.
Figure 5. Nystatin activity against fluconazole-resistant on non-albicans Candida.Nystatin activityagainst fluconazole-resistantn on-albicans Candida
Nystatin is equally effective on fluconazole-susceptible or -resistant strains. The nystatin mode MIC and MIC range on a collection of 32 strains with MIC >=4 mg/l (17 of them MIC >=8 mg/l) was identical to that corresponding to the susceptible strains. No nystatin resistance was found among this group of resistant strains, and the absence of cross-resistance was again documented (Fig. 5).
Activity of nystatin compared with amphotericin B
Nystatin is less active than amphotericin B, but the range of distribution is shorter in the case of nystatin: 1 to 8 mg/l compared with 0.03 to 4 mg/l for amphotericin B. The reasons for this high dispersion of amphotericin B MICs are probably multifactorial and remain largely unknown (Fig. 6).
Cross-resistance amongazole compounds in C. albicans
One important part of the interest in the reevaluation of the polyenic compounds is to spare the use of azole compounds in order to decrease the selective power of these drugs for resistant strains. The relevance of cross-resistance among azole compounds remains debatable; in any case, according to our own data on more than 130 strains, the problem of cross-resistance does indeed occur. Strains with fluconazole MICs >=64 mg/l have significantly higher MICs to ketoconazole (>=4 mg/l) or itraconazole (= 8 mg/l) (p <0.001). On the other hand, strains with ketoconazole MICs = 2 mg/l have increased MICs to itraconazole (>=8 mg/l) (p = 0.028). Thus, the problem is real; discrepancies may occur in some cases, but may be due to the technical conditions of the assays more than to real differences in mechanisms of resistance.
Figure 6. Activity of nystatin compared with amphotericin B.Rare cross-resistance between azoleand polyenic compounds
Several clinical observations support the practical absence of cross-resistance between azole and polyenic compounds. AIDS patients with thrush due to fluconazole -resistant C. glabrata, and unresponsive to fluconazole treatment were cured with amphotericin B (203). On the contrary, amphotericin-resistant invasive hepatosplenic candidiasis was controlled by fluconazole therapy (247); nystatin resistant C. albicans oral infections was succesfully treated with miconazole (248). Interestingly, an AIDS patient with Candida esophagitis under treatment with fluconazole developed resistance to this drug, and amphotericin B treatment was started; but a new failure occurred. In necropsy, the strain was recovered and proved to have an increased amphotericin B MIC (249)Controversy exists because of these cases.
In a case of esophageal candidiasis with azole and polyene resistance, there was clinical failure of amphotericin B therapy in an HIV-infected patient with known fluconazole resistance (249).
Importance of the diminution of the intestinal reservoir
The human gastrointestinal tract is an important reservoir for Candida (250), and most Candida infections originate in this area, either directly (local infection or invasive spread after persorption) or indirectly (skin contamination by intestinal content). Therefore, in susceptible patients (typically immunosuppressed), preventive strategies have been considered in order to reduce or eliminate Candida from the gut. Both polyene and azole agents have been used for this a purpose in selective decontamination protocols (251) in association with antibacterial agents (252-258). Many of these protocols are capable of a very significant reduction in the yeast content of the gut (257, 259). A similar efficacy was found for nystatin and ketoconazole (260). Interestingly, patients with severe undernutrition (a relevant problem in the hospital setting or in elderly people) frequently carry Candida in the stomach. In a study, 65% of patients of this type had Candida in this localization; this high rate might have been increased by the use of gastric tubes, favoring the transit of Candida from the oral reservoir to the stomach (261). Again, this and other studies of paired oral and gastroduodenal aspirate cultures suggest that identifying Candida in the oral cavity is a good indicator of the presence of yeasts elsewhere in the gastrointestinal tract. On the other hand, mechanisms through which overgrowth of Candida in the upper gastrointestinal tract might contribute to the inflammatory background of peptic ulcer disease have also been suggested (262).
Optimal dosing and galenic aspects: absorption and distribution Nystatin
Oral suspension. Nystatin oral suspension is usually administered at 100,000 U/ml. Some authors recommend topical nystatin at a concentration of 150,000 U/ml three times a day for 14 to 21 days. In some studies carried out in neonatal (263) or elderly patients, a preparation was used, mixing 48 ml of oral nystatin suspension with 432 ml of distilled water, which reaches a solution containing about 10,000 IU/ml of nystatin ml. This solution was used together with the oral preparation (vaginal tablets, 100,000 IU/ g), and administered three t.i.d. for a week (36). In general, in the prophylaxis and treatment of oropharyngeal candidiasis in newborns, 1 ml of the oral suspension is instilled in each side of the mouth every 4 h. In premature infants, 0.5 ml is recommended. In adults, the recommended schedule is 2 to 3 ml in each side of the mouth q.i.d.
Tablets. Nystatin tablets contain 500,000 U, with the average dosage being 1,000,000 U to 1,500,000 U/day for children and 2,000,000 to 5,000,000 U/day for adults, in four or six administrations (264). Tablets may be sucked or chewed four times/day. Due to the peculiar, unpleasant taste of the tablet and oral suspension, many patients prefer vaginal tablets or ovules that are used in an identical way (192). Tablets and oral suspension have identical efficacy, both from the microbiological or clinical point of view (265, 266). In cases of esophagitis, the clinical response may be dependent on the dose and frequency of administration. Maximal doses of 100,000 U/h to 1,000,000 U.I. q.i.d. have been used (267). The association with methylcellulose (0.5%) or carboxymethylcellulose (0.7%) prolongs the period of mucosal contact. It is essential to remember that nystatin is an antifungal agent which acts by contact and that prolonged interaction with the mucosa is essential.
Vaginal ovules. Ovules, primarily focused to control vulvovaginal candidosis, contain 100,000 U of nystatin in combination with lactose, ethylcellulose, stearic acid and starch. Ovules are administered once or twice/day for 2 weeks.
Ointment. Ointment preparation, to be used in skin Candida infections, contains 100,000 U/g and should be applied twice a day for prophylactic treatment of burns.
Liposome-encapsulated nystatin. This was studied in vitro (268) and in vivo for systemic fungal infections. Liposome encapsulation provides a means for intravenous administration of nystatin, reducing its toxicity and making it an active systemic antifungal agent (269).
Absorption and distribution
By the oral route, nystatin is practically nonabsorbed; most of the drug is eliminated in the feces (177); during the first 24 h about one-third of nystatin is recovered in this sample, and less than 1% in urine (270). In any case it is important to remember that bioessay determinations of nystatin in feces are not very sensitive (40 U.I./gram is the detection limit) (271). If the expected fecal excretion rate is one-third in 24 h, a certain irregularity may occur from dose to dose and patient to patient, and the distribution in the intestinal tract is heterogeneous (263). Obviously the decrease in the intestinal motility may delay the transit of nistatin, and may reduce effectiveness: this is the case for premature and low-weight newborns when compared with normal infants (272). Despite high intestinal concentrations, there is no effect on intestinal bacterial flora (273).
After extremely high oral doses (10 million units), blood concentrations of 1 to 2.5 mg/l have been detected. These concentrations are not expected to produce any significant antifungal effect and are devoid of toxicity (274).
POLYENE DRUG INTERACTIONS IN IMMUNOSUPPRESSED PATIENTS: THE DIFFERENCES FROM AZOLES
Nystatin should not be mixed with chlorhexidine in the local therapy of oral infection; the interaction may result in a decrease of solubility (275). Fluconazole, at doses 200 mg/day, produce significant interactions with rifampicin (276), zidovudine (277) and cyclosporin. These drugs are frequently used in immunosuppressed patients and during organ transplantation (278-280). The clinical relevance of the interaction of fluconazole with polyenes remains controversial. A frequently repeated concern is the inhibition of ergosterol synthesis by azoles, reducing the possible interaction of the polyenes with this compound, which could result in a certain drug antagonism. Despite these concerns, it has become apparent that the combined use of amphotericin B and fluconazole is a common occurrence in medical practice around the world. As has been said, the interaction of azoles and polyenes may vary with the fungus, test models and drug. The conclusion is that it is unlikely that an inclusive general rule concerning the concomitant or sequential use of these drugs to cover all situations will be forthcoming (281).
CONCLUSION
The value of nystatin as a prophylactic anfifungal agent should be reviewed. The safety and efficacy of nystatin has been proven for nearly half a century, and the resistance of yeasts to polyene compounds has been maintained in most areas of the world during this period of time. New antifungal agents such as the azole compounds have a key role in the current chemotherapy for deep fungal infections. However, resistance has emerged leading to the danger of spreading resistant yeasts which will require a change in the therapeutic and preventive strategies. Since azole use should be probably spared to reduce the intensity of selection, and control of health care costs is a progressively increasing factor in medical decisions, nystatin may have a renewed position in future chemotherapy of fungal infections, with a specific use as a prophylactic strategy. New approaches towards Candida strain resistance need to be developed and encouraged.
ACKNOWLEDGEMENTS
This work was supported by Grupo Bristol-Meyers Squibb España.
BIBLIOGRAFÍA
- Hippocrates, ca 460-377 BC. In: Epidemics, book 3. Williams and Wilkins, Baltimore 1939.
- Langenbeck, B. Auffingung von Pilzen aus der Schleimhaut der Speiseröhre einer Typhus-Leich. Neue Not Geb Natur u Heilk (Froriep) 1839; 12: 145-147.
- Rippon, J.W. Candidosis. The pathogenic yeasts. In: Medical mycology. W.B. Saunders, Philadelphia 1974; 181.
- Pereiro Miguens, M., Pereiro Ferreiros, M. Candidosis cutaneomucosas. In: Masson, S.S. (Ed.). Micología médica 1993; 131-143.
- Lehner, T. Classification and clinico-pathological features of Candida infection in the mouth. In: Winner, H., Hurley, R. (Eds.). Symposium on Candida infections. E & S. Livingstone, Ltd., Edinburgh and London 1966; 119-137.
- Cutler Juan, E. Putative virulence factors of Candida albicans. Annu Rev Microbiol 1991; 45: 187-218.
- Rippon, J.W. Candidiasis and the pathogenic yeasts. In: Medical mycology. W.B. Saunders Company, Philadelphia 1988; 531.
- Ramanathan, K., Han, N.K., Chelvanayagam, P.I. Oral candidiasis � Its pleomorphic clinical manifestations, diagnosis and treatment. Dent J Malays 1985; 8: 39-45.
- Zegarelli, D.J. Fungal infections of the oral cavity. Otolaryngol Clin North Am 1993; 26: 1069-1089.
- Ben-Aryeh, H., Blumfield, E., Szargel, R., Laufer, D., Berdicevsky, I. Oral Candida carriage and blood group antigen secretor status. Mycoses 1995; 38: 355-358.
- Mackay, B.J., Denepitiya, L., Iacono, V.J., Krost, S.B., Pollock, J.J. Growth-inhibitory and bactericidal effects of human parotid salivary histidine-rich polypeptides on Streptococcus mutans. Infect Immun 1984; 144: 695-701.
- Pollock, J.J., Denepitiya, L., MacKay, B.J., Iacono, V.J. Fungistatic and fungicidal activity of human parotid salivary histidine-rich polypeptides on Candida albicans. Infect Immun 1984; 44: 702.
- Aly, F.Z., Blackwell, C.C., MacKenzie, D.Z. et al. Chronic atrophic oral candidiasis among patients with diabetes mellitus-role of secretor status. Epidemiol Infect 1991; 106: 355-363.
- Lamey, P.J., Darwazeh, A.M., Muirhead, J. et al. Chronic hyperplastic candidosis and secretor status. J Oral Pathol Med 1991; 20: 64.
- Nikawa, H., Kotani, H., Sadamori, S., Hamada, T. Denture stomatitis and ABO blood types. J Prosthet Dent 1991; 66: 391-394.
- Thom, S.M., Blackwell, C.C., MacCallum, C.J., Weir, D.M. et al. Non-secretion of blood group antigens and susceptibility to infection by Candida species. FEMS Microbiol Immunol 1989; 1: 401-405.
- Cohen, R., Roth, F.J., Delgado, E., Ahearn, D.G., Kalser, M.H. Fungal flora of the normal human small and large intestine. New Engl J Med 1969; 280: 638-641.
- Martin, M.V., Lamb, D.J. Frequency of Candida albicans serotypes in patients with denture-induced stomatitis and in normal denture wearers. J Clin Pathol 1982; 35: 881-891.
- Arendorf, T.M., Walker, D.M. Oral candidal populations in health and disease. Br Dent J 1979; 147: 267-272.
- Hauman Ch., Thompson, I.O., Theunissen, F., Wolfaardt, P. Oral carriage of Candida in healthy and HIV-seropositive persons. Oral Surg Oral Med Oral Pathol 1993; 76: 570-572.
- Schmitt, J.A. Epidemiological investigations of oral Candida albicans. Mycopath Mycol Applicata 1977; 43: 65.
- Burnett, O.W., Scherp, H.W. In: Oral microbiology and infections disease, 3rd ed. Williams & Wilkins, Baltimore 1968.
- Arendorf, T.M., Walker, D.M. The prevalence and intra-oral distribution of Candida albicans in man. Arch Oral Biol 1980; 25: 1-10.
- Epstein, J.B., Pearsall, N.N., Truelove, E.J. Quantitative relationships between Candida albicans in saliva and the clinical status of human subjects. J Clin Microbiol 1980; 12: 475-476.
- Williamson, J.J. Diurnal variation of Candida albicans counts in saliva. Aust Dent J 1972; 17: 54-60.
- Mitchell, K.G., Bradley, J.A., Ledinghan, I.M., Hamilton, D.N.H. Candida colonization of the oral cavity. Surg Gynecol Obstet 1982; 154: 870-874.
- Kauffman, C.A., Jones, P.G. Candidiasis (a diagnostic and therapeutic challenge). Posgrad Med J 1986; 180: 129-133.
- Odds, F.C. Candida species and virulence. ASM News 1994; 60: 313-318.
- Rindum, J.L., Stenderup, A., Holmstrup, P. Identification of Candida albicans types related to healthy and pathological oral mucosa. J Oral Pathol Med 1994; 23 (9): 406-412.
- Bastiaa, R.J., Reade, P.C. The prevalence of Candida albicans in the mouths of tobacco smokers with and without oral mucous membrane keratoses. Oral Surg Med Oral Pathol 1982; 53: 148-151.
- Darlin, M.R., Arendorf, T.M., Coldrey, N.A. Effect of cannabis use on oral candidal carriage. J Oral Pathol Med 1990; 19: 319-321.
- Odds, F.C. Ecology of Candida and epidemiology of candidosis. In: Candida and candidosis, 2nd ed. Bailliere Tindall, London 1988; 68-92.
- Rippon, J.W. Candidosis. The pathogenic yeasts. In: Medical mycology. WB Saunders Company, Philadelphia 1974; 179.
- Matee, M.I., Simon, E., Christensen, W., Kirk, K., Andersen, L., Samaranayake, L.P., Scheutz, F. Association between carriage of oral yeasts and malnutrition among Tanzanian infants aged 6-21 months. Oral Dis 1995; 1: 37-42.
- Wilkieson, C., Samaranayake, L.P., MacFarlane, T.W., Lamey, P.J., MacKenzie, D. Oral candidosis in the elderly in long term hospital care. J Oral Pathol Med 1991; 20: 1.
- Banting, D.W., Greenhorn, P.A., McMinn, J.G. Effectiveness of a topical antifungal regimen for the treatment of oral candidiasis in older, chronically ill, institutionalized, adults. J Can Dent Assoc 1995; 61: 199-200, 203-205.
- Mathaba, L.T., Davies, G., Warmington, J.R. The genotypic relationship of Candida albicans strains isolated from the oral cavity of patients with denture stomatitis. J Med Microbiol 1995; 42: 372- 379.
- Ceballos, A., Gonzales Moles, M., Urquia, M. Dentadure stomatitis. Its relation to Candida albicans. Av Odontoestomatol 1990; 6: 151- 154.
- Cawson, R.A. Chronic oral candidosis, denture stomatitis and chronic hyperplastic candidosis. In: Winner, H.I., Hurley, R. (Eds.). Symposium on Candida infections. Livingstone, Edinburgh 1966; 138-153.
- Devenport, J.C. The oral distribution of Candida in denture stomatitis. Br Dent J 1970; 129: 51-56.
- Budtz Jörgensen, E. Clinical aspects of Candida infection in denture wearers. J Am Dent Assoc 1978; 96: 474-479.
- Blixt Johansen, G., Sjoeholm, Wiesel, K., Ek, A.C. The condition of the oral mucosa in institutionalized elderly patients before and after using a mucin-containing saliva substitute. Scan J Caring Sci 1992; 6147-6150.
- Wagner, D.K., Sohnie, P.G. Cutaneous defenses against Dermatophytes and yeasts. Clin Microbiol Rev 1995; 8: 317-335.
- Shepherd, M.G., Poulter, R.T.M., Sullivan, P.A. Candida albicans: Biology, genetics and pathogenicity. Ann Rev Microbiol 1985; 39: 579-614.
- Epstein, J.B., Komiyama, K., Duncan, D. Oral topical steroids and secondary oral candidiasis. J Oral Pathol Med 1986; 41: 223-227, 273.
- Schechtman, R.L., Archard, H.O., Cox, D. Oropharyngeal candidiasis associated with steroid-containing inhalers. NY State Dent J 1986; 52: 24-26.
- Lehrer, R., Cline, M. Interaction of Candida albicans with human leukocytes and serum. J Bacteriol 1969; 98: 996-1004.
- Odds, F.C. Factor that predispose the host to candidosis. In: Candida and candidosis, 2nd ed. Bailliere Tindall, London 1988.
- Thompson, H.L., Wilton, J.M.A. Interaction and intracellular killing of Candida albicans blastospores by human polymorphonuclear leucocytes, monocytes and monocyte-derived macrophages in aerobic and anaerobic conditions. Clin Exp Immunol 1992; 87: 316-321.
- Lockhart, P.B., Sonis, S.T. Alterations in the oral mucosa caused by chemotherapeutic agents. J Dermatol Surg Oncol 1981; 7: 1019- 1025.
- Brown, L.R., Driezen, S., Handler, S., Johnston, D.A. Effect of radiation-induced xerostomia on human oral microflora. J Dent Res 1975; 54: 740-750.
- Rosie, K.M., Taylor, J., Beck, F.M., Hodgson, S.E., Blozis, G.G. Influence of radiation therapy on oral Candida albicans colonization: A quantitative assement. Oral Surg Oral Med Oral Pathol 1987; 64: 698-701.
- Epstein, J.B., Vickars, L., Spinell, J., Reece, D. Efficacy of chlorhexidine and nystatin rinses in prevention of oral complications in leukemia and bone marrow transplantation. Oral Surg Oral Med Oralpathol 1992; 73: 682-689.
- Trifonava, L.I., Smirnova, T.A., Volozhin, A.L. Tissue changes in the oral cavity of children with primary immunodeficiency states. Stomatologika Mosk 1993; 72: 73-75.
- Galán, S.F., García, M.P., Marín, C.P., Mira, G.P. Yeasts in oropharynx of immunocompromised patients. In: Congress of European Confederation of Medical Mycology, Lisboa 1996; Abstr. 7.3.
- Epstein, J.B., Freilich, M.M., Le, N.D. Risk factors for oropharyngeal candidiasis in patients who receive radiation therapy for malignant conditions of the head and neck. Oral Surg Oral Med Oral Pathol 1993; 76: 169-174.
- Kramer, B.K., Pizzo, P.A., Robichaud, K.J. et al. Role of the serial microbiological surveillance and clinical evaluation in the management of cancer patients with fever and granulocytopenia. Am J Med 1982; 72: 561-568.
- Childer, N.K., Stinnett, E.A., Wheeler, P., Wright, J.T., Castleberry, R.P., Dasanayake, A.P. Oral complications in children with cancer. Oral Surg Ond Med Oral Pathol 1993; 75: 41-47.
- Okamoto, K., Ryoke, K., Hamada, T. Isolation of oral Candida and inununological parameters for head and neck. Yonago Acta Med 1994; 37: 195-203.
- Meyers, J.D., Thomas, E.D. Infection complicating bone marrow transplantation. In: Young L.S., Ruben, R.H. (Eds.). Clinical approach to infection in the compromised host. Plenum Medical Company, New York 1981; 507-551.
- Dreizen, S., Bodey, G.P., Rodríguez, V. Oral complications of cancer chemotherapy. Postgrad Med 1975; 58: 75-82.
- Dreizen, S., McCredie, K.B., Keating, M.J., Bodey, G.P. Oral infections associated with chemotherapy. Postgrad Med 1982; 71: 133-146.
- McElroy, T.H. Infection in the patient receiving chemotherapy for cancer: Oral considerations. J Am Dent Assoc 1984; 109: 454-456.
- Sürücüoglu, S., Hilmioglu, S., Türker, M., Özbakkaloglu, B., Baran. Oral and intestinal Candida colonization in patients with hematologic malignancy and the susceptibility of the isolated strains to nystatin. Turk J Infect 1993; 7: 351-352.
- O'Sullivan, E.A., Duggal, M.S., Bailey Curzon, M.E., Hart, P. Changes in the oral microflora during cytotoxic chemotherapy in children being treated for acute leukemia. Oral Surg Oral Med Oral Pathol 1993; 76: 161-168.
- Carl, W. Oral and dental care of patients receiving chemotherapy. In: Carl, W., Sako, K. III. (Eds.). Cancer and the oral cavity. Quitessense Publishing Co., Inc., Chicago 1986; 151-165.
- Peterson, D.E., Sonis, S.T. Oral complications of cancer chemotherapy: Present status and future studies. Cancer Treat Rep 1983; 66: 1251-1256.
- Brown, L.R., Dreien, S., Bodey, G.P. The effect of immunosuppression on the human flora. In: Mergenhagen, S.E., Scherp, H.W. (Eds.). Comparative immunology of the oral cavity. US Government Printing Office, Washington DC 1973; 204-220.
- Quirk, P.C., Osborne, P.J., Walsh, L.J. Australian Dental Research Fund Trebitsch Scholarship. Efficacy of antifungal prophylaxis in bone marrow transplantation. Aust Dent J 1995; 40: 267-270.
- Berkowitz, R., Hughes, C., Rudnick, M. et al. Oropharyngeal Candida prophylaxis in pediatric bone marrow transplant patients. Am J Pediatr Hematol Oncol 1985; 7: 82-86.
- Viviane, M.A., Tortorano, A.M., Malasoina, C. et al. Surveillance and treatment of liver transplant recipients for candidiasis and aspergillosis. Eur J Epidemiol 1992; 8: 433-436.
- Gupta, K.I., Ghosh, A.K., Kochhar, R., Jha, V., Chakrabarti, A., Sakhuja, V. Esophageal candidiasis after renal transplantation: Comparative study in patients on different inununosuppressive protocols. Am J Gastroenterol 1994; 89: 1062-1065.
- Lunch, E., Albert, J., Linde, A. et al. Human immunodeficiency virus type I and cytomegalovirus in saliva. J Med Virol 1993; 39: 156-162.
- Glatt, A.E., Chirgwin, K., Landesmann. Treatment of infections associated with immunodeficiency virus. N Engl J Med 1988; 318: 1439-1448.
- Budtz Jorgensen, E. Etiology, pathogenesis, therapy, and prophylaxis of oral yeast infections. Acta Odontol Scand 1990; 48: 61-69.
- Marchisio, P., Principi, N. Treatment of oropharyngeal candidiasis in HIV-infected children with oral fluconazole. Multicentre Study Group. Eur J Clin Microbiol Infect Dis 1994; 13: 338-340.
- Sweet, S.P., Cookson, S., Challacombe, S.J. Candida albicans isolates from HIV-Infected and AIDS patients exibit enhanced adherence to epithelial cells. J Med Microbiol 1995; 43: 452-457.
- Pollock, J.J., Santarpia, R.P., Heller, H.M. et al. Determination of salivary anticandidal activities in healthy adults and patients with AIDS: A pilot study. J Acquir Inmune Defic Syndr 1995; 5: 610-618.
- Barchiesi, F., Giacometti, A., Arzeni, D. et al. Fluconazole and ketoconazole in the treatment of oral and esophageal candidiasis in AIDS patients. J Chemother 1992; 4: 381-386.
- Lewis, M.A., Samaranyake, L.P., Lamey, P.J. Diagnosis and treatment of oral candidosis. J Oral Marillofac Surg 1991; 49: 996-1002.
- Stevens, D.A., Greene, S.I., Lang, O.S. Thrush can be prevented in patients with acquired immunodeficiency syndrome and the acquired immunodeficiency syndrome-related complex. Arch Intern Med 1991; 151: 2458-2464.
- Berthold, P., Stewart, J., Cumming, C., Decker, S., Macgregor, R., Malamud, D. Candida organisms in dental plaque from AIDS patients. J Infect Dis 1994; 170: 1053-1054.
- Nieto García, A., Guix García, J., Navarro Ibáñez, V., Roig Rico, P., Bemacer Alpera, B. Role of oral candidiasis as a predictive marker of tuberculosis in patients with HIV infection. An Med Intern 1992; 9: 318-217.
- Moniaci, D., Cavallari, M., Greco, D. et al. Oral lesions in children born to HIV-1 positive women. J Oral Pathol Med 1993; 22: 8-11.
- Franker, C.K., Lucartorto, F.M., Johnson, B.S., Jacobson, J.J. Characterization of the mycoflora from oral mucosal surfaces of some HIV-infected patients. Oral Surg Oral Med Oral Pathol 1990; 69: 683-687.
- Torssander, J., Morfeldt Manson, L., Biberfeld, G., Karlsson, A., Putkonen, P.O., Wasserman, J. Oral Candida albicans in HIV infection. Scan J Infect Dis 1987; 19: 291-295.
- Fetter, A., Partsani, M., Koenig, H., Kremer, M., Lang, J.M. Asymptomatic oral Candida albicans carriage in HIV-infection: Frequency and predisposing factors. J Oral Pathol Med 1993; 22: 57-59.
- Lamey, P.J., Darwaza, A., Fisher, B.M., Samaranayake, L.P., Mac-Farlane, T.W., Frier, B.M. Secretor status, candidal carriage and candidal infection in patients with diabetes mellitus. J Oral Pathol 1988; 17: 354-357.
- Blackwell, C.C., Aly, F.Z., James, V.S., Weir, D.M., Collier, A., Patrick, A.W., Cumming, C.O., Wray, D., Clarke, B.F. Blood group, secretor status and oral carriage of yeast among patients whit diabetes mellitus. Diabetes Res 1989; 12: 101-104.
- Bartholomew, G.A., Rodu, B., Bell, D.S. Oral candidiasis in patients with diabetes mellitus: A thorough analysis. Diabetes Care 1987; 10: 607-612.
- Ozturkcan, S., Ozturkcan, S., Topeu, S., Akinci, S., Bakici, M.Z., Yalcin, N. Incidence of oral candidiasis in diabetic patients. Mikrobiyol Bul 1993; 27; 352-356.
- Darwazeh, A.M., Lamey, P.J., Samaranayake, L.P. et al. The relationship between colonisation, secretor status and in vitro adhesion of Candida albicans to bucal epithelial cells from diabetics. J Med Microbiol 1990; 33: 43-49.
- Clotet, B., Grifol, M., Parra, O. et al. Asymptomatic esophageal candidiasis in the acquired-immunodeficiency-syndrome-related complex. Ann Inter Med 1986; 105: 145.
- Ismail, A., Abdulla, S. Pos-monilial extensive esophageal structure. Pediatr Hematol Oncol 1993; 10: 111-113.
- Wilcox, C.M., Straub, R.F., Clark, W.S. Prospective evaluation of oropharyngeal findings in human immunodeficiency virus-infected patients with esophageal ulceration. AMJ Gastroenterol 1995; 90: 1938-1941.
- Hay, R.H. Overview of studies of fluconazole in oropharyngeal candidiasis. Rev Infect Dis 1990; 12 (Suppl. 3): 334-337.
- López Dupla, M., Mora Sanz, P., Pintado García, V. et al. Clinical, endoscopic, immunologic, and therapeutic aspects of oropharyngeal and esophageal candidiasis in HIV-infected patients: A survey of 114 cases. AMJ Gastroenterol 1992; 87: 1771-1776.
- Fotos, P.G., Hellstein, J.W. Candida and candidosis. Epidemiology, diagnosis and therapeutic management. Dent Clin North Am 1992; 36: 857-878.
- Egger, T., Gratwohl, A., Tichelli, A. et al. Comparison of fluconazole with oral polyenes in the prevention of fungal infections in neutropenic patients. A prospective, randomized, single-center study. Support Care Cancer 1995; 3: 139-146.
- Guiot, H.F.L., Fibbe, W.E., van't Wout, J.W. Risk factors for fungal infection in patients with malignant hematologic disorders: Implications for empirical therapy and prophylaxis. Clin Infect Dis 1994; 18: 525-532.
- Grant, S.M., Clissold, S.P. Fluconazole: A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in superficial and systemic mycoses. Drugs 1990; 39: 877-916.
- Como, J.A., Dismukes, W.E. Oral azole drugs as systemic antifungal therapy. N Engl J Med 1994; 330: 263-272.
- Bodey, G.P. Azole antifungal agents. Clin Infect Dis 1992; 14 (Suppl. 1): 161-169.
- Hitchcock, C.A., Barret Bee, K.J., Rusell, N.J. The lipid composition and permeability to azole of an azole and poliene-resistant mutant of Candida albicans. J Med Vet Micol 1987; 25: 29-37.
- Vanden Bossche, H., Marichal, P., Gorrens, J., Bellens, D., Moereels, H., Janssen, P.A.J. Mutations in cytochrome P450-dependent 14-demethylase result in decreased affinity for azole antifungals. Biochem Soc Trans 1990; 18: 56-59.
- Van den Bossche, H., Marichal, P., Odds, F.C. Molecular mechanisms of drug resistance in fungi. Trends Microbiol 1994; 2: 393-400.
- Horne, J., Hollomon, D., Loeffler, R.S.T., Kelly, S.L. Altered P450 activity associated with direct selection for fungal azole resistance. FEBS Letter 1995; 374: 174-178.
- Ishida, N., Aoyama, Y., Hatanaka, R. et al. A single amino acid substitution converts cytochrome P450 (14 DM) to an inactive form, cytochrome P450 (SGI) complete primary structures deduced from cloned DNAs. Biochem Biophys Res Commun 1988; 155: 317-323.
- Kenna, S., Bligh, H.F.J., Watson, P.F., Kelly, S.L. Genetic and physiological analysis of azole sensitivity in Saccharomyces cerevisiae. J Med Vet Mycol 1989; 27: 397-406.
- Bard, M., Lees, N.D., Barbuch, R.J., Sanglard, D. Characterisation of a cytochrome P450 deficient mutant of Candida albicans. Biochem Biophys Res Commun 1987; 147: 794-800.
- Van den Bossche, H., Marichal, P., Odds, F.C., Le Jeune, L., Coene, M.C. Characterization of an azole-resistant Candida glabrata isolate. Anti Agent Chem 1992; 36: 2602-2610.
- Warnock, D.W., Burke, J., Cope, N.J., Johnson, E.M., von Fraunhofer, N.A., Williams, E.W. Fluconazole resistance in Candida glabrata. Lancet 1988; ii: 1310.
- Kelly, S.L., Rowe, J., Watson, P.F. Molecular genetic studies on the mode of action of azole antifungal agents. Biochem Soc Trans 1991; 19: 796-798.
- Watson, P.F., Rose, M.E., Ellis, S.W., England, H., Kelly, S.L. Defective sterol C5-6 desaturation and azole resistance. A new hypothesis on the mode of action of azole antifungal agents. Biochem Biophys Res Commun 1989; 164: 1170-1175.
- Kelly, S.L., Kelly, D.E. Molecular studies on azoles sensitivity in fungi. In: Maresca, B., Kobayashi, G.S., Yamaguchi, H. (Eds.). Molecular biology and its application to medical mycology. Springer Verlag, Berlin 1993; 199-213.
- Kelly, S.L., Lamb, D.C., Corran, A.J., Baldwing, B.C., Kelly, D.E. Mode of action and resistance to azole antifungals asociated with the formation of 14a-methylergosta-8,24(28)-dien-3,6a-diol. Biochem Biophys Res Commun 1995; 207: 910-915.
- Kelly, S.L., Lamb, D.C., Kelly, D.E., Loeffler, J., Einsele, H. Resistance to fluconazole and amphotericin in Candida albicans from AIDS patients. Lancet 1996; 348: 1523-1524.
- Kelly, S.L., Lamb, D.C., Kelly, D.E. et al. Resistance to fluconazole and cross-resistance to amphotericin B in Candida albicans from AIDS patients caused by defective sterol delta 5,6-desaturation. FEBS Lett 1997; 400: 80-82.
- Parkinson, T., Falconer, D.J., Hitchcock, C.A. Fluconazole resistance due to energy-dependent drug efflux in Candida glabrata. Antimicrob Agents Chemother 1995; 39: 1696-1699.
- Sanglard, D., Kuchler, K., Ischer, F., Pagani, J.L., Monod, M., Bile, J. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve especific multidrug transporter. Antimicrob Agents Chemother 1995; 39: 2378-2386.
- Venkateswarlu, K., Denning, D.W., Manning, N.J., Kelly, S.L. Resistance to fluconazole in Candida albicans from AIDS patients correlated with reduced intracellular accumulation of drug. FEMS Microbiol Lett 1995; 131: 337-341.
- Sanglard, D., Ischer, F., Monod, M., Bille, J. Cloning of Candida albicaw genes conferring resistance to azole antifungal agents: Characterization of CDR2, a new multidrug ABC transporter gene. Microbiology 1997; 143: 405-416.
- Law, D., Moore, C.B., Wardle, H.M., Ganguli, L.A., Keaney, M.G., Denning, D.W. High prevalence of antifungal resistance in Candida spp. from patients with AIDS. J Antimicrob Chemother 1994; 34: 659-668.
- Marichal, P., Gorrens, J., Coene, M.C., LeJeune, L., Vanden Bossche, H. Origin of differences in susceptibility of Candida krusei to azole antifungal agents. Mycoses 1995; 38: 111-117.
- Venkateswarlu, K., Denning, D.W., Maning, N.J., Kelly, S.L. Reduced accumulation of drug in Candida krusei accounts for itraconazole resistance. Antimicrob Agents Chemother 1996; 40: 2443-2446.
- Venkateswarlu, K., Denning, D.W., Kelly, S.L. Inhibition and interaction of sterol 14-demethylase of Candida krusei with azole antifungal drugs. J Med Vet Mycol 1997; 35: 19-25.
- Johnson, E.M., Warnock, D.W., Luker, J., Porter, S.R., Scully, C. Emergence of azole drug resistance in Candida species from HIV-infected patients receiving prolonged fluconazole therapy for oral candidosis. J Antimicrob Chemother 1995; 35: 103-114.
- Just Nuebling, G., Gentschew, G., Meissner, K. et al. Fluconazole prophylaxis of recurrent oral candidiasis in HIV-positive patients. Eur J Clin Microbiol Infect Dis 1991; 10: 917-921.
- Reents, S., Goodwin, S.D., Singh, V. Antifungal prophylaxis in immunocompromised hosts. Ann Pharmacother 1993; 27: 53-60.
- Thunnissen, P.L., Sizoo, W., Hendriks, W.D. Safety and efficacy of itraconazole in prevention of fungal infections in neutropenic patients. Neth J Med 1991; 39: 84-91.
- Sanguineti, A., Carmichael, J.K., Campbell, K. Fluconazole-resistan Candida albicans after long-term suppressive therapy. Arch Intern Med 1993; 153: 1122-1124.
- Harkness, J., Marriott, D., Fulton, W. et al. An open assessment of the safety and efficacy of oral fluconazole in the treatment of oropharyngeal candidiasis in HIV infected patients. Aust N Z J Med 1990; (Suppl. 20): 436.
- Sarnow, E., Baranowski, E., Bauer, O. et al. Oropharyngeal candidiasis in HIV patients. Fluconazole in the treatment in registered practice. Munch Med Wochenschr 1993; 134: 85-87.
- Plettenberg, A., Stoehr, A., Höffken, G. et al. Fluconazole therapy of oral candidiasis in HIV-infected patients: Results of a multicentre study. Infection 1994; 22: 118-123.
- Meunier, F., Aqun, M., Noriega, M., Janssens, M., Phan Thi, M. Fluconazole versus ketoconazole dans le traitement des candidoses oropharyngees et oesophagiennes chez des patients atteints de cancer. J Hycol Med 1991; 11 (Suppl. 1): 14-19.
- Powderly, W.G., Finkelstein, D.M., Feinberg, J. et al. A randomized trial comparing fluconazole with clotrimoxazole troches for the prevention of fungal infections in patients with advanced human immunodeficiency virus infection. NIAID-AIDS Clinical Trial Group. N Engl J Med 1995; 332: 700-705.
- Hitchcock, C.A. Azole antifungal resistance in Candida albicans. Apua Newsletter 1996; 14: 1-58.
- Georgopapadakou, N.H., Walsh, T.J. Antifungal Agents: Chemotherapeutic targets and immunologics strategies. Antimicrob Agents Chemother 1996; 40: 279-291.
- Pons, V., Greenspan, D., Debruin, M. Therapy for oropharyngeal candidiasis in HIV-infected patients: A randomized, prospective multicenter study of oral fluconazole versus clotrimazole troches. The multicenter study group. J Acquir Immune Defic Syndr 1993; 6: 1311-1316.
- Gearhart, M.O. Worsening of liver function with fluconazole and review of azole antifungal hepatotoxicity. Ann Pharmacother 1994; 28: 1177-1181.
- Fromtling, R. Overview of medically important azole derivatives. Clin Microbiol Rev 1988; 1: 187-217.
- Georgopapadakou, N.H., Dix, B.A., Smith, S.A., Freudenberger, J., Funke, P.T. Effect of antifungal agents on lipid biosynthesis and membrane integrity in Candida albicans. Antimicrob Agents Chemother 1987; 31: 46-51.
- Sud, I.J., Feingold, D.S. Action of antifungal imidazoles in Staphylococcus aureus. Antimicrob Agents Chemother 1982; 22: 470-474.
- Hazen, E., Brown, R. Two anfifungal agents produced by a soil actinomycete. Science 1950; 112: 423.
- Dutcher, J.D., Boyack, G., Fox, S. The preparation and properties of crystalline fungicidin (nystatin). Antibiot Annal 1953- 1954: 191.
- Gold, W., Stout, H.A., Pagano, J.F, Donovick, R. Amphotericin A and B antifungal antibiotics produced by a Streptomycete. I. In vitro studies. Antibiot Annu 1956; 1955-56; 579-586.
- Dutcher, J.D. The discovery and development of amphotericin B. Dis Ches 1968; 54: 40-42.
- Amphotericin B. In: McEvoy, G.K. (Ed.). American Hospital Formulary Service Drug Information. American Society of Hospital Pharmacists, Bethesda, Md 1988; 71-74.
- E.R. Squibb & Sons, Inc. Fungizone intravenous prescribing information. In: Oradell, N.J. (Ed.). Physicians'desk reference, 42nd. Medical Economics 1988; 2052-2053.
- Sokol-Anderson, M.L., Brajtburg, J., Medoff, G. Sensitive of Candida albicans to amphotericin B administered as single of fractionated doses. Antimicrob Agents Chemother 1986; 29: 701-702.
- Stein, S.H., Little, J.R., Little, K.D. Parallel inheritance of tissue catalase activity and inmunostimulatory action of amphotericin B in inbred mouse straind. Cell Inmunol 1987; 105: 99-109.
- Lamy Freund, M.T., Ferreira, V.N.F., Schreier, S. Mechanism of inactivation of the polyene antibiotic amphotericin B: Evidence for radical formation in the process of autooxidation. J Antibiot 1985; 38: 753-757.
- Brajtburg, J., Powderly, W.G., Kobayashi, G.S., Medoff, O. Amphotericin B: Current understanding of mechanisms of action. Antimicrob Agents Chemother 1990; 34: 183-188.
- Whyte, B.S., Peterson, R.P., Hartsel, S.C. Amphotericin B and nystatin show different activities on sterol-free vesicles. Biochem Biophys Res Comm 1989; 164: 609-614.
- Hamilton Miller, J.M.T. The effect of pH and temperature on the stability and bioactivity of nystatin and amphotericin B. J Pharm Pharmacol 1973; 25: 401-407.
- Van't Wout, J. Fungal infections and antifungal drugs: Has the age of antifungal resistance dawned? Curr Opin Infect Dis 1996; 9: 63-65.
- Hebeka, E.K., Solotorovsky. Development of resistance to polyene antibiotic of Candida albicans. J Bacteriol 1965; 89: 1533-1539.
- Conly, J., Renin, R., Johson, J. et al. Disseminated candidosis due to amphotericin-B resistant Candida albicans. J Infect Dis 1992; 165: 761-764.
- Dick, J.D., Merz, W.G., Saral, R. Incidence of polyene-resistant yeasts recovered from clinical specimens. Antimicrob Agents Chemother 1980; 18: 158-163.
- Dick, J.D., Rosengard, B.R., Merz, W.G., Stuart, R.K., Hutchins, G.M., Saral, R. Fatal disseminated candidiasis due to amphotericin B resistant Candida guilliermondi. Ann Intern Med 1985; 102: 68-69.
- Drutz, D.J., Lehrer, R.I. Development of amphotericin B-resistant Candida tropicalis in a patient with defective leukocyte function. Am J Med Sci 1978; 276: 77-92.
- Merz, W.G., Sandfor, G.R. Isolation and characterization of a poliene-resistand variant of Candida tropicalis. J Clin Microbiol 1979; 9: 677-680.
- Pappagianis, D., Collins, M.S., Hector, R., Remington, J. Development of resistance to amphotericin B in Candida lusitaniae infecting a human. Antimicrob Agents Chemother 1979; 16: 123-126.
- Korting, H.C., Oller, M., Georgii, A., Froschl, M. In vitro susceptibilities and biotypes of Candida albicans isolates from the oral cavities of patients infected with human immunodeficiency virus. J Clin Microbiol 1988; 26: 2626-2631.
- Powderly, W.G., Kobayashi, G.S., Herzig, G.P., Medoff, G. Amphotericin B-resistant yeast infection in severely immunocompromised patients. Am J Med 1988; 84: 826-832.
- Gale, E.F. Nature and development of phenotypic resistance to amphotericin B in Candida albicans. Adv Microb Physiol 1986; 27: 278-320.
- Pierce, A.M., Pierce, A.M. Jr., Unrau, H.D., Oehlschlager, A.M. Lipid composition and poliene antibiotic resistance of Candida albicans. Can J Biochem 1978; 56: 135-142.
- Athar, M.A., Winner, H.I. The development of resistance by Candida species to polyene antibiotics in vitro. J Med Microbiol 1971; 4: 505-517.
- Ghannoum, A.M., Swairjo, I., Soll, D.R. Variation in lipid and sterol contents in Candida albicans white and opaque phenotypes. J Med Vet Mycol 1990; 28: 103-115.
- Sokol-Anderson, I.M.L., Sligh, J.E., Elberg, S., Brajtburg, J., Kobayashi, G.S., Medoof, G. Role of cell defense against oxidative damage in the resistance of Candida albicans. To the killing effect of amphotericin B. Antimicrob Agents Chemother 1988; 32: 702-705.
- Broughton, M.C., Bard, M,, Lees, N.D. Polyene resistance in ergosterol producing strains of Candida albicans. Mycoses 1991; 34: 75-83.
- Woods, R., Ahmed, K.A. Genetically controlled cross resistance to polyene antibiotics in Saccharomyces cerevisiae. Nature 1968; 218: 1080-1084.
- Dubé, M.P., Heseltine, P.N., Rinaldi, M.G., Evans, S., Zawacki, B. Fungemia and colonization with nystatin-resistant Candida rugosa in a burn unit. Clin Infect Dis 1994; 18: 77-82.
- Hazen, E.L., Brown, R. Fungicidin, an antibiotic produced by a soil actinomycete. Proc Soc Exp Biol 1951; 76-93.
- Drouhet, E. Traitement des infections mycosiques à Candida albicans par un nouvel antibiotique antifongique, la nystatine. Presse Méd 1955; 63: 620.
- Drouhet, E. Resistance des champignons aux antibiotiques antifongiques. Path Biol 1967; 15: 1223-1226.
- Drouhet, E. Antifungal agents. Antibiotics Chemother 1978; 25: 253-288.
- Hussain Qadr, S.M., Flournoy, D.J., Qadri, S.G., Ramírez, E.G. Susceptibility of clinical isolates of yeasts to antifungal agents. Mycopathologia 1986; 95: 183-187.
- Nierebinska, E., Gwiezdzinski, Z., Urbanowski, S., Pracz, M. Phenomenon of increased resistance of yeast-like fungi to nystatin. Wiad Lek 1992; 45: 427-429.
- Mete, M., Arikan, E. Oral and intestinal Candida colonization in antibiotic-treated children and in vitro susceptibility of Candida species to nystatin. Turk J Infect 1993; 7: 115-119.
- Meunier, F., Paesmans, M., Autier, P. Value of antifungal prophylaxis with antifungal drugs against oropharyngeal candidiasis in cancer patients. Eur J Cancer B Oral Oncol 1994; 30B (3): 196-199.
- Mayer, K.H., DeTorres, O.H. Current guidelines on the use of antibacterial drugs in patients with malignancies. Drugs 1985; 29: 262-279.
- Buchanan, A.G., Riben, P.D., Rayner, E.N., Parker, S.E., Ronald, A.R., Louie, T.J. Nystatin prophylaxis of fungal colonization and infection in granulocytopenic patients: Correlation of colonization and clinical outcome. Clin Invest Med 1985; 8: 139-147.
- Prentice, A.G. Oral and gastrointestinal candidosis: Prophylaxis during immunosuppressive therapy. Mycoses 1989; 32 (Suppl. 2): 42-46.
- Ferretti, G.A., Brown, A.T., Raybould, T.P., Lillich, T.T. Oral antimicrobial agents, chlorhexidine. NCI Monogr 1990; 9: 51-55.
- Winston, D.J., Ho, W.G., Bruckner, D.A., Gale, R.P., Champlin, R.E. Ofloxacin versus vancomycin-polymyxin for prevention of infections in granulocytopenic patients. Am J Med 1990; 88: 36-42.
- Hoppe, J.E., Friess, D., Niethammer, D. Orointestinal yeast colonization of paediatric oncologic patients during antifungal prophylaxis: Results of quantitative culture and Candida serology and comparison of three polyenes. Mycoses 1995; 38: 41-49.
- Vogler, W.R., Malcom, L.G., Winton, E.F. A randomized trial comparing ketoconazole and nystatin prophylactic therapy in neutropenic patients. Cancer Investigation 1987; 5: 267-273.
- Ruskin, J.D., Wood, R.P., Bailey, M.R., Whitmore, C.K., Shaw, B.W. Comparative trial of oral clotrimazole and nystatin for oropharyngeal candidiasis prophylaxis in orthotopic liver transplant patients. Oral Surg Oral Med Oral Pathol 1992; 74: 567-571.
- MacPhail, L.A., Hilton, J.T., Dods, C.L., Greenspan, D. Prophylaxis with nystatin pastillas for HIV-associated oral candidiasis. J Acquir Immune Defic Syndr Hum Retrovirol 1996; 12: 470-476.
- Wurtele, P. Treatment of ear and mouth mycosis. J Otolaryngol 1987; 16: 191-2.40.
- John, E.E. Muguet de la boca y del esófago. In: Enfermedades infecciosas. Braude. Médica Panamericana, 1988; 50.
- Poland, J.M. Oral thrush in the oncologic patient. Therapy must be tailored. Am J Hosp Care 1987; 4: 30-32.
- Spiechowicz, E., Santarpia, P., Pollock, J., Renner, R.P. In vitro study on the inhibiting effect of different agents on the growth of Candida albicans on acrylic resin surfaces. Quintessence Int 1990; 21: 35-40.
- Truhlar, M.R., Shay, K., Sohnle, P. Use of a new assay technique for quantification of antifungal activity of nystatin incorporated in denture liners. J Prosthet Dent 1994; 71: 517-524.
- Lewis, M.A., Meechan, C., MacFarlane, T.W., Lamey, P.J., Kay, E. Presentation and antimicrobial treatment of acute orofacial infections in general dental practice. Br Dent J 1989; 166: 41-45.
- Lever, S.A., Dupuis, L.L., Chan, H.S. Comparative evaluation of benzydamine oral rinse in children with antineoplastic-induced stomatitis. Drug Intell Clin Pharm 1987; 21: 359-361.
- Rothwell, B.R., Spektor, W.S. Palliation of radiation-related mucositis. Spec Care Dentist 1990; 10: 21-25.
- Meunier, F., Gerain, J., Snoeck, R. Oral treatment of oropharingeal candidiasis with nystatin versus ketoconazole in cancer patients, a randomized study. Drug Invest 1990: 2: 71-75.
- Boon, J.M., Lafeber, H.N., Mannetje, A.H. et al. Comparison of ketoconazole suspension and nystatin in the treatment of newborns and infants with oral candidosis. Mycoses 1989; 32: 312-315.
- Nyst, M.J., Perriens, J.H., Kimputo, L., Lumbila, M., Nelson, A.M., Piot, P. Gentian violet, ketoconazole and nystatin in oropharyngeal and esophageal candidiasis in Zairian AIDS patients. Ann Soc Belg Med Trop 1992; 72: 45-52.
- Flynn, P.M., Cunningham, C.K., Kerkering, T. et al. Oropharingeal candidiasis in immunocompromised children: A randomized, multicenter study of orally administered fluconazole suspension versus nystatin. The multicenter fluconazole study group. J Pediatr 1995; 127: 322-328.
- Dewsnup, D.H., Stevens, D.A. Efficacy of oral amphotericin B in AIDS patients with thrush clinically resistant to fluconazole. J Med Vet Mycol 1994; 32: 389-393.
- Cosey, R.J. Contac dermatitis due to nystatin. Arch Dermatol 1971; 103: 228.
- Wasilewski, C. Allergic contact dermatitis from nystatin. Arch Dermatol 1970; 102: 216-217.
- Lechner, T., Grytzmann, B., Baurle, G. Hematogenous allergic contact dermatitis after oral administration of nystatin. Mykosen 1987; 30: 143-146.
- Holeman, C.W., Einstein, H. The toxic effects of amphotericin B in man. California Med 1963; 99: 90-93.
- Kinsky, S.C., Avruch, J. et al. The lytic-effect of polyene antifungal antibiotics on mammalian erythrocytes. Biochem Biophys Res Commun 1962; 9: 503-507.
- Kinsky, S.C. Antibiotic interaction with model membranes. Annu Rev Pharmacol 1970; 119-142.
- Adler Moore, J.P., Proffitt, R.T. Development, characterization, efficacy and mode of action of AmBisome, a unilamellar liposomal formulation of amphotericin B. J Liposomal Res 1993; 3: 429-450.
- Francis, P., Lee, J.W., Hoffman, A. et al. Efficacy of unilamellar liposomal amphotericin B in treatment of pulmonary aspergillosis in persistently granulocytopenic rabbits. J Infect Dis 1994; 169: 356-368.
- López Barestein, G., Bodey, G.P., Fainstein, V. et al. Treatment of systemic fungal infections with liposomal amphotericin B. Arch Inter Med 1989; 149: 2533-2536.
- Ringden, O., Meunier, E., Tollemer, J. et al. Efficacy of amphotericin B encapsulated in liposomes (AmBisome) in the treatment of invasive fungal infections in immunocompromised patients. J Antimicrob Chemother 1991; 28 (Suppl. B): 73-82.
- Meunier, F. New methods for delivery of antifungal agents. Rev Infect Dis 1989; 11 (Suppl. 7): 1605-1609.
- Hanson, L.H., Stevens, D.A. Comparison of the antifungal activity of amphotericin B deoxycholate suspension with that of amphotericin cholesteryl sulfate colloidal dispersion. Antimicrob Agents Chemother 1992; 36: 486-488.
- MacPhreson, D.T., Corbett, D.F., Costello, B.C. et al. Adventures in polyene macrolide chemistry: The derivatization of amphotericin B. In: Bently, P.H., Ponsford, R. (Eds.). Recent advances in the chemistry of anti-infective agents. Royal Society of Chemistry, London 1993; 205-222.
- Holt, R.J., Aznii, A. Miconazole-resistant Candida. Lancet 1978; i: 50-51.
- Rex, J.H., Rinaldi, M.G., Pfaller, M.A. Resistance of Candida species to fluconazole. Antimicrob Agents Chemother 1995; 39: 1-8.
- Hoepelman, I.M., Dupont, B. Oral candidiasis: The clinical challenge of resistance and management. Int J Antimicrob Agents 1996; 6: 155-159.
- Diz Dios, P.D., Álvarez Álvarez, J., Fernández Feijoo, J., Castro Ferreiro, M. Fluconazole response patterns in HIV-infected patients with oropharyngeal candidiasis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1995; 79: 170-174.
- Horn, C.A., Washburn, R.G., Givner, L.B., Peacock, J.E., Pegram, P.S. Azole-resistant oropharyngeal and esophageal candidiasis in patients with AIDS. AIDS 1995; 9: 533-535.
- Millon, L., Manteaux, A., Reboux, G. et al. Fluconazole-resistant recurrent oral candidiasis in human immunodeficiency virus positive patients: Persistence of Candida albicans strains with the same genotype. J Clin Microbiol 1994; 32: 1115-1118.
- Hay, R.J. Antifungal therapy of yeast infections. J Am Acad Dermatol 1994; 31: S-6-9.
- Maenza, J.R., Keruly, J.C., Moore, R.D., Chaisson, R.E., Merz, W.G., Gallant, J.E. Risk factors for fluconazole resistant candidiasis in human immunodeficiency virus-infected patients. J Infect Dis 1996; 173: 219-225.
- Polanco, A.M., Martínez-Suárez, J.V., Rodríguez-Tudela, J.L. Resistance of Candida spp. to fluconazole in AIDS patients. Enferm Infec Microbiol Clin 1993; 11: 462-464.
- García Hermoso, D., Dromer, F., Improvisi, L., Provost, F., Dupont, B. Fluconazole concentrations in saliva from AIDS patients with oropharyngeal candidosis refractory to treatment with fluconazole. Antimicrob Agents Chemother 1995; 39: 656-660.
- Barchiesi, F., Hollis, R.J., Poeta, M. et al. Transmission of fluconazole-resistant Candida albicans between patients with AIDS and oropharyngeal candidiasis documented by pulsed-field gel electrophoresis. Clin Infect Dis 1995; 21: 561-564.
- Fan Havard, P., Capano, D., Smith, S.M., Mangia, A., Eng, R.H. Development of resistance in Candida isolates from patients receiving prolonged antifungal therapy. Antimicrob Agents Chemother 1991; 35: 2302-2305.
- Redding, S., Smith, J., Farinacci, G., Rinaldi, M. et al. Resistance of Candida albicans to fluconazole during treatment of oropharyngeal candidiasis in a patients with AIDS: Documentation by in vitro susceptibility testing and DNA subtype analysis. Clin Infect Dis 1994; 18: 20-22.
- Ruhnke, M., Eigler, A., Tennagen, I., Geiselor, B., Engelman, E., Trautmann. Emergence of fluconazole-resistant strains of Candida albicans in patients with recurrent oropharyngeal candidosis and human immunodeficiency virus infection. J Clin Microbiol 1994; 32: 2092-2098.
- Rodríguez Tudela, J.L., Martínez Suárez, J.V., Dronda, F., Laguna, F., Chaves, F., Valencia, E. Correlation of in vitro susceptibility test results with clinical response: A study of azole therapy in AIDS patients. J Antimicrob Chemother 1995; 35: 793-804.
- Barchiesi, F., Hollis, R.J., McCough, D.A., Scalise, G., Rinaldi, M.G., Pfaller, M.A. DNA subtypes and fluconazole susceptibilities of Candida albicans isolates from the oral cavities of patients with AIDS. Clin Infect Dis 1995; 20: 634-640.
- McCullough, M., Hinne, S. A longitudinal study of the change in resistance patterns and genetic relationship of oral Candida albicans from HIV-infected patients. J Med Vet Mycol 1995; 33: 33-37.
- Quereda, C., Polanco, A.M., Giner, C. et al. Correlation between in vitro resistance to fluconazole and clinical outcome of oropharyngeal candidiasis in HIV-infected patients. Eur J Clin Microbiol Infect Dis 1996; 15: 30-37.
- Millon, L., Reboux, G. Emergence de Candida glabrata et Candida krusei chez des patients séropositifs pour le VIH atteints de candidose oropharyngée, traités de façon prolongée par le fluconazole. J Mycol Med 1994; 4: 90-92.
- Grapipin, M., Buisson, M., Duong, M. et al. Change in Candida susceptibility to fluconazole in HIV patients after termination of secondary systematic prophylaxis for oropharyngeal candidosis. Méd Malad Infect 1995; 25: 32-39.
- Wingard, J.R., Merz, W.G., Rinaldi, M.G., Jhonson, T.R., Karp, J.E., Saral, R. Increase in Candida krusei infection among patients with bone marrow transplantation and neutropenia treated prophylactically with fluconazole. N Engl J Med 1991; 325: 1274-1277.
- Philpott Howard, H.N., Wade, J.J., Mufti, G.J., Brammer, K.W., Ehninger, G. Randomized comparison of oral fluconazole versus oral polyenes for the prevention of fungal infection in patients at risk of neutropenia. J Antimicrob Chemother 1993; 31: 973-984.
- Greenspan, D. Treatment of oropharyngeal candidiasis in HIV-positive patients. J Am Acad Dermatol 1994; 31: S51-S55.
- Dhondt, F., Ninane, J., De Beule, K., Dhondt, A., Cauwenbergh, G. Oral candidosis: Treatment with absorbable and non-absorbable antifungal agents in children. Mycoses 1992; 35: 1-8.
- Dupont, B. Azole antifungal agents: Emerging and inherent resistance. Curr Opin Infect Dis 1995; 8: 424-427.
- Odds, F.C. Review: Resistance of yeast to azole-derivative antifungals. J Antimicrob Chemother 1993; 31: 463-471.
- National Commitee for Clinical Laboratory Standars. Reference method for broth dilution antifungal susceptibility testing for yeast. Proposed Standard M-27P. NCCLS, Villanova, Pa 1992.
- Espinel-Ingroff, A., Kish, Jr. C.W., Kerkering, T.M. et al. Collaborative comparison of broth macrodilution and microdilution antifungal susceptibility test. J Clin Microbiol 1992; 30: 3138-3145
- Espinel-Ingroff, A., Steele Moore, L., Galgiani, J.N. Evaluation of 80% inhibition standars for the determination of fluconazole minimum inhibitory concentrations in three laboratories. Diagn Microbiol Infect Dis 1994; 20: 81-86.
- Anaissie, E.F., Karyotakis, N.C., Hachem, R. et al. Correlation between in vitro and in vivo activity of antifungal agents against Candida species. J Infect Dis 1994; 170: 384-389.
- Jakab, K., Keleman, E., Prinz, G., Torok, Y. Amphotericin-resistant invasive hepatosplenic candidiasis controlled by fluconazole. Lancet 1990; 335: 473-474.
- Martin, M.V., Dindale, R.C.W. Nystatin-resistance of Candida albicans isolates from two cases of oral candidiasis. Br J Oral Surg 1982; 20: 294-298.
- Le Thong, P., Tuazon Carmelita, U., Levine, M., Borum, M., Rollhauser, C. Resistance to fluconazole and amphotericin B in a patient with AIDS who was beging treated for candidal esophagitis. Clin Infect Dis 1996; 23: 649-650.
- Meunier, F. Prevention of mycosis in immunocompromised patients. Rev Infect Dis 1987; 9: 408-416.
- Damjanovic, V., Connolly, C.M., Van Saene, H.K. et al. Selective decontamination with nystatin for control of a Candida outbreak in a neonatal intensive care unit. J Hosp Infect 1993; 24: 245-259.
- Rhodes, L.E., van Saene, H.K., White, S., Fairclough, S., Ball, L.M., Martin, J. Microbial carriage, sepsis, infection and acute GVHD in the first 25 BMT at the Royal Liverpool Children's Hospital. Bone Marrow Transplant 1993; 11: 261-269.
- Baranov, A.E., Nadezhina, N.M., Petrosian, L.N. et al. Prevention of infectious complications in patients with acute bone marrow depression. Antibiot-Khimioter 1991; 36: 38-40.
- Nagao, T., Yonekura, S., Komatsuda, M. et al. Clinical significance of gastrointestinal decontamination under protected environment. Kansenshogaku-Zasshi 1991; 65: 40-46.
- Flaherty, J., Nathan, C., Kabins, S.A., Weinstein, R.A. Pilot trial of selective decontamination for prevention of bacterial infection in an intensive care unit. J Infect Dis 1990; 162: 1393-1397.
- Murdoch, D.A., Gibbs, S., Price, C.G. et al. Effect of ceftazidime and gentamicin on the oropharyngeal and faecal flora of patients with haematological malignancies. J Antimicrob Chemother 1990; 26: 419-428.
- Ferrer, M., Torres, A., Gonzales, J. et al. Utility of selective digestive decontamination in mechanically ventilated patients. Ann Intern Med 1994; 120: 389-395.
- Ramounau Pigot, A., Monnet, P., Boyer, G., Darbas, H., Donadio, D. Prevention des septicemies d'origine digestive chez les granulopeniques: Comparaison de trois traitements de decontamination digestive. Pathol Biol Paris 1991; 39: 131-135.
- Bar, W., Welling, G.W., Kurrle, E. Effects of selective oral antimicrobial prophylaxis and systemic antibiotics on the fecal flora and fecal beta-aspartylglycine concentration in patients with acute leukemia. APMIS 1989; 97: 705-714.
- Cortellaro, M., Cofrancesco, E., Pasargiklian, I. et al. Ciprofloxacin for infection prophylaxis in granulocytopenic patients with acute leukemia. Haematologica 1990; 75: 541-545.
- Gottlieb, K., Iber, F.L., Livak, A., Leya, J., Mobarhan, S., Hines, E. Oral candida colonizes the stomach and gastrostomy feeding tubes. J Parenter Enteral Nutri 1994; 18: 264-267.
- Burford Mason, A.P., Willoughby, J.M., Weber, J.C. Association between gastrointestinal tract carriage of Candida, blood group O, and nonsecretion of blood group antigens in patients with peptic ulcer. Dig Dis Sci 1993; 38: 1453-1458.
- Blaschke Hellmessen, R., Pannwitz, U., Schwarze, R. Intestinal excretion of nystatin during and after oral administration to newborn infants at risk. Monatsschr Kinderheilkd 1991; 139: 89-91.
- Medoff, G., Kobayashi, G.A. The polyenes. In: Speller, D.C.E. (Ed.). Antifungal chemotherapy. John Wiley & Sons 1980; 3-33.
- Thompson, P.J., Wingfield, H.J., Cosgrove, R.F., Hughes, B.O., Turner Warwick, M.E. Assessment of oral candidiasis in patients with respiratory disease and efficacy of a new nystatin formulation. Br Med J Res Ed 1986; 292 (6537): 1699-1700.
- Johnson, G.H., Taylor, T.D., Heid, D.W. Clinical evaluation of a nystatin pastille for treatment of denture-related oral candidiasis. J Prosthet Dent 1989; 61: 699-703.
- Mathieson, R., Dutta, S.K. Candida esophagitis. Dig Dis Sci 1983; 28: 365-370.
- Mehta, R.T., Hopfer, R., Gunner, L.A., Juliano, R.L., López Berestein, G. Formulation, toxicity, and antifungal activity in vitro of liposome-encapsulated nystatin as therapeutic agent for systemic candidiasis. Antimicrob Agents Chemother 1987; 36: 1897-1900.
- Mehta, R.T., Hopfer, R., McQueen, T., Juliano, R.L., López Berestein, G. Toxicity and therapeutic effects in mice of liposome- encapsulated nystatin for systemic fungal infections. Antimicrob Agents Chemother 1987; 31: 1901-1903.
- Drouhet, E. Antifungal antibioties: Biological activity. In: Wolstenholme, G.E.W., Porter, R. (Eds.). Ciba Foundation Symposium on Systemic Mycoses. J & A. Churchill Ltd., London 1968; 206-224.
- Blaschke Hellmessen, R., Schwarze, U. Bioassay for determination of nystatin in faeces after oral application. Mycoses 1990; 33: 129-135.
- Blaschke Hellmessen, R., Schwarze, R. Mycologic monitoring of newborn infants at risk during preventive administration of nystatin. Monatsschr-Kinderheilkd 1991; 139: 92-95.
- Sande, M.A., Mandell, G.L. Antimicrobial agents. Miscellaneous antibacterial agents; antifungal and antiviral agents. In: Gilman, A.G., Goodman, L., Gilman, A. (Eds.). The pharmacological basis of therapeutics, 6th ed. Macmillan, 1980; 1232-1240.
- Kucers, A., Mc Bennett, N. Nystatin. In: The use of antibiotic. A comprehensive review with clinical emphasis, 2nd ed. William Heinemann Medical Books, London 1975; 601-603.
- Barkvoll, P., Hurlen, B. Konvensjonell behandling av orale candidoser; enkelte fallgruber. NorTanntaegeforen Tid 1989; 99: 116- 119.
- Aseloff, G., Hilligoss, D.M., Gardner, M.J. et al. Induction of fluconazole metabolism by rifampin: In vivo study in humans. J Clin Pharmacol 1991; 31: 358-361.
- Sahai, J., Gallicano, K., Pakuts, A. Effect of fluconazole on zidovudine pharmacokinetics in patients infected with human immunodeficiency virus. J Infect Dis 1994; 169: 1103-1107.
- Canafax, D.M., Graves, N.M., Hilligoss, D.M. et al. Interaction between cyclosporine and fluconazole in renal allograft recipients. Transplantation 1991; 51: 1014-1018.
- Muñoz Sanz, A., Cubero, J., Caravaca, F. et al. Interaction between ciclosporine and fluconazole in a renal transplant. Rev Esp Quimioterap 1992; 5: 352-353.
- López Gil, J.A. Fluconazole-cyclosporine interaction: A dose-dependent effect? Ann Pharmacother 1993; 27: 427-430.
- Alan, M. Sugar. Use of amphotericin B with azole antifungal drugs: What are we doing? Antimicrob Agents Chemother 1995; 39: 1907- 1912.